AP Chemistry Unit 4.1: Introduction to Reactions
Written by SlyAcademy
Table of Contents
- What is a Chemical Reaction?
- Physical vs. Chemical Changes
- How are Chemical Reactions Represented?
- Types of Chemical Reactions
- Practice Questions
- Conclusion
What is a Chemical Reaction?
In the realm of AP Chemistry, a chemical reaction is a process that involves the rearrangement of atoms, resulting in the creation of new substances. Reactions form the backbone of many everyday phenomena, from how our devices function to the fizzy bubbles in soda.
Physical vs. Chemical Changes
When discussing matter, there are two primary types of changes: physical changes and chemical changes.
Physical Change: A change that alters an object’s state or appearance without affecting its chemical composition. For example, boiling water transforms liquid water into steam, but the molecular structure remains H2O. Similarly, shredding a piece of paper changes its form but not its chemical identity.
Chemical Change: This change results in the formation of new substances with distinct chemical properties. For example, iron (Fe) reacts with oxygen (O2) to form rust (iron oxide, Fe2O3). Similarly, mixing baking soda (NaHCO3) with vinegar (CH3COOH) releases carbon dioxide and produces new substances.
Indicators of Chemical Changes:
- Heat or Light: Often observed in combustion reactions.
- Formation of Gas: Noted by bubbling or odor release.
- Precipitation: Formation of a solid when two solutions are mixed.
- Color Change: Certain reactions change color based on pH or other factors.
How are Chemical Reactions Represented?
Chemical reactions are expressed through chemical equations. Here’s how to read and understand these equations:
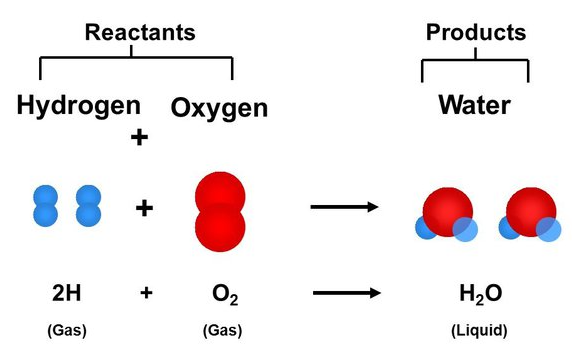
Image Courtesy of Quora
- Reactants (left side): Substances that undergo change.
- Products (right side): Substances formed as a result of the reaction.
- Arrow (→): Indicates the direction of the reaction.
- Coefficients: Represent the relative number of molecules or moles involved. For example, in 2H2 + O2 → 2H2O, the coefficient “2” shows that two water molecules are produced.
Example Breakdown: In H2O, “H” represents hydrogen atoms and “O” represents oxygen atoms. The subscript “2” shows there are two atoms of hydrogen in each molecule.
Types of Chemical Reactions
Understanding the different types of reactions provides a foundation for mastering AP Chemistry.
Synthesis Reactions
Definition: Two or more reactants combine to form a single product.
- General Formula: A + B → AB
- Example: 2Na + Cl2 → 2NaCl (Sodium and chlorine combine to form sodium chloride).

The Synthesis of Sodium Chloride from Sodium Metal and Chlorine Gas
Decomposition Reactions
Definition: A single compound breaks down into two or more products.
- General Formula: AB → A + B
- Example: 2H2O2 → 2H2O + O2 (Hydrogen peroxide decomposes into water and oxygen).

The Elephant’s Toothpaste: A violent reaction that is caused by a speeding up of the decomposition of hydrogen peroxide using a catalyst
Combustion Reactions
Definition: Organic molecules (containing carbon) combust in the presence of oxygen, forming carbon dioxide and water while releasing energy.
- General Formula: Hydrocarbon + O2 → CO2 + H2O
- Example: CH4 + 2O2 → CO2 + 2H2O (Combustion of methane).
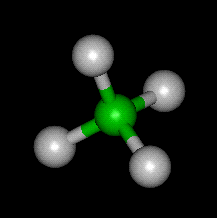
The Methane Molecule – Image Courtesy of world of molecules
Single Replacement Reactions
Definition: An element reacts with a compound, replacing one component.
- General Formula: AB + C → AC + B
- Example: 3Mg + 2AlCl3 → 3MgCl2 + 2Al (Magnesium replaces aluminum).
Double Replacement Reactions
Definition: Ions in two compounds exchange places, forming new compounds.
- General Formula: AB + CD → AD + CB
- Example: HCl + NaOH → NaCl + H2O (Acid-base reaction producing water and salt).
Practice Questions
Identify the reaction type:
- Zn + 2HCl → ZnCl2 + H2
- Answer: Single Replacement
- 2C8H18 + 25O2 → 16CO2 + 18H2O
- Answer: Combustion
- 2H2O → 2H2 + O2
- Answer: Decomposition
- AgNO3 + NaCl → AgCl + NaNO3
- Answer: Double Replacement
- 2Ni2O3 → 4Ni + 3O2
- Answer: Decomposition
- 2Na + Cl2 → 2NaCl
- Answer: Synthesis
- Cl2 + 2NaBr → 2NaCl + Br2
- Answer: Single Replacement
- BaCl2 + Na2SO4 → BaSO4 + 2 NaCl
- Answer: Double Replacement
- C3H8 + 5O2 → 3CO2 + 4H2O
- Answer: Combustion
Conclusion
Mastering chemical reactions, their types, and how they are represented is crucial for success in AP Chemistry. From synthesis to combustion, understanding these foundational concepts will prepare you for more complex topics and deepen your appreciation of the chemical processes shaping our world.







