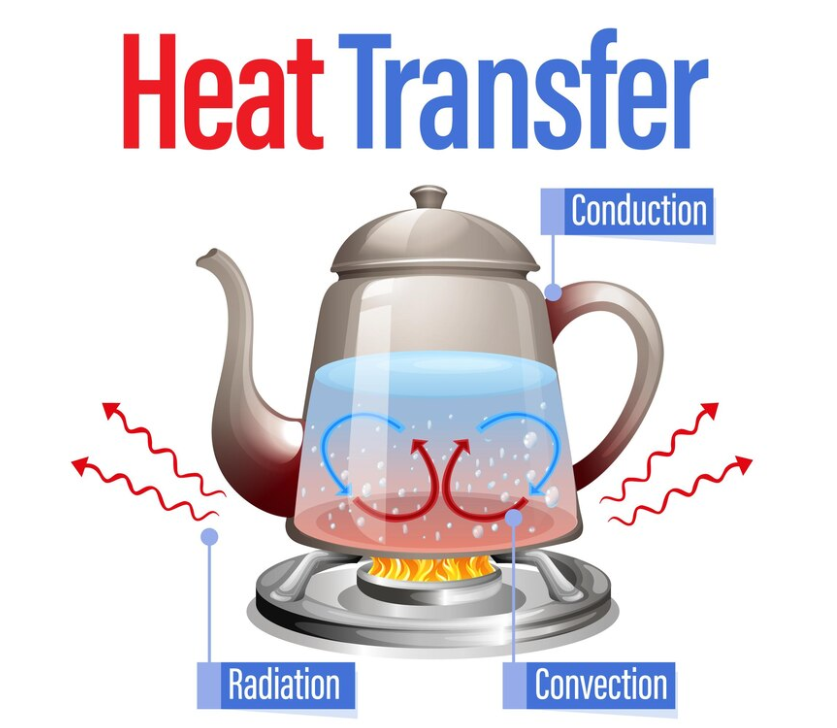
6.3 Heat Transfer and Thermal Equilibrium
Understanding Heat Transfer and Molecular Collisions
In chemistry, temperature is a measure of the average kinetic energy of particles in a substance. As temperature increases, molecules move faster and collide more frequently with the walls of their container. This random motion is at the core of how energy is transferred, particularly in the form of heat.
The Basics of Collision Theory
Collision theory explains how and why chemical reactions occur. For a reaction to take place, reactant molecules must collide with:
- Sufficient Energy: Collisions must have enough energy to break bonds and overcome the activation energy barrier.
- Proper Orientation: Molecules must collide in the correct position for bonds to break and new bonds to form.
Key Idea:
Successful collisions = Proper orientation + Enough energy
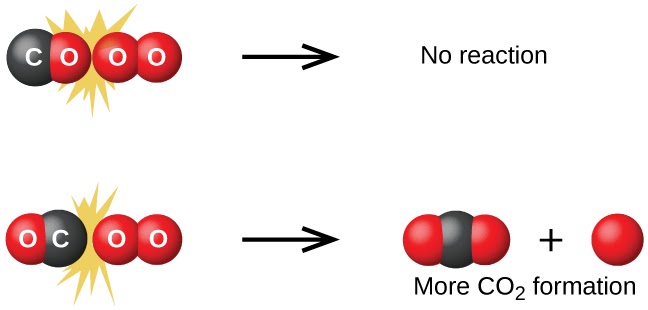
Image Courtesy of Chemistry BC Textbook
Analogy: Imagine making lemonade by mixing lemon juice, sugar, and water. Just having the ingredients isn’t enough—they need to be mixed correctly. Similarly, molecules need the right collision energy and orientation to react.
Increasing the temperature or concentration of reactants speeds up reactions by increasing the frequency and energy of molecular collisions. This is why heat transfer is so crucial in reaction kinetics.
Heat Transfer and Thermal Equilibrium
How Heat Moves Between Objects
Heat transfer naturally occurs from a hot object (source) to a cold object (sink) until both reach the same temperature—this state is known as thermal equilibrium. At thermal equilibrium, the molecules of both objects are moving at the same speed, indicating equal temperatures.
Real-Life Example:
When a hot pan 🍳 is placed on a stove’s heated grates, heat flows from the grates to the pan until both are at the same temperature. This process illustrates how heat travels from a higher-temperature body to a lower-temperature one.
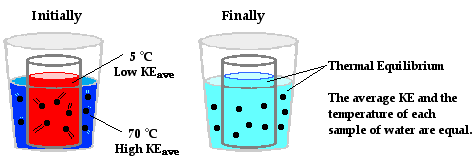
Image Courtesy of ThePhysicsClassroom
The Zeroth Law of Thermodynamics
The Zeroth Law of Thermodynamics states:
“If object A is in thermal equilibrium with object B, and object B is in thermal equilibrium with object C, then object A is in thermal equilibrium with object C.”
This law emphasizes the fundamental nature of thermal equilibrium and serves as the foundation for temperature measurement and heat transfer analysis.
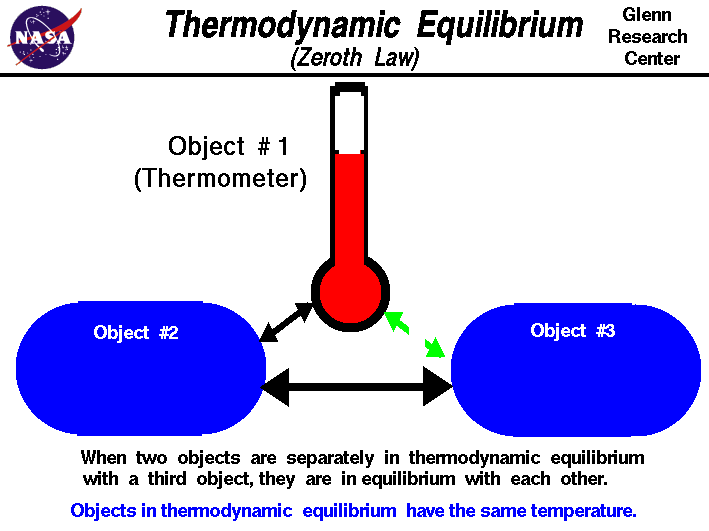
Image Courtesy of NASA
Practical Implications of Heat Transfer
Temperature and Collisions
As molecules heat up, their kinetic energy increases, leading to faster-moving particles and more frequent collisions. This energy transfer is crucial for understanding how chemical reactions proceed and how heat conduction works. Higher temperatures lead to faster molecular motion and more successful collisions, driving reactions forward.
Quick Takeaways:
- Temperature reflects the average kinetic energy of particles.
- Collision theory requires sufficient energy and proper orientation for reactions.
- Heat transfer occurs from hot to cold objects until thermal equilibrium is reached.
- The Zeroth Law of Thermodynamics establishes a fundamental rule about thermal equilibrium between objects.
Explore More:
- Understanding Energy Transfer in Reactions
- Collision Theory and Reaction Kinetics
- Thermodynamics in Everyday Life
Mastering the concepts of heat transfer and thermal equilibrium deepens your understanding of how energy moves and reacts in chemical processes—an essential skill for any chemistry student. Keep learning and stay curious!