Solutions: Aqueous Chemistry and Beyond
When examining a chemical reaction, the four phases of matter you may encounter after each compound are: (s) solid, (l) liquid, (g) gas, or (aq) aqueous. So far, we’ve explored solids, liquids, and gases in-depth. Now, it’s time to delve into solutions — particularly those labeled with (aq), meaning “aqueous,” or dissolved in water.
Review of Mixtures
In AP Chemistry, mixtures typically refer to heterogeneous mixtures, where the macroscopic properties depend on the location of the particles in the mixture. An example of a heterogeneous mixture is soil 🌱, where you can see the distinct components that make up the mixture.
On the other hand, homogeneous mixtures are uniform in composition, meaning the macroscopic properties are consistent throughout the sample. For example, saltwater is a homogeneous mixture — you cannot see the individual components like the salt and water once the salt has dissolved.
Example of Homogeneous Mixture:
- Saltwater: the salt is completely dissolved and uniformly mixed in water.
What are Solutions?
Solutions are a type of homogeneous mixture where the particles are evenly distributed, and the solute is uniformly dissolved within the solvent. The solute is the substance that dissolves, while the solvent is the substance doing the dissolving.
Examples of Solutions:
Solid Solutions (Alloys):
- Steel: Solute = carbon, Solvent = iron.
- Brass: Solute = zinc, Solvent = copper.
Liquid Solutions:
- Saltwater: Solute = salt, Solvent = water.
- Lemonade: Solutes = sugar, lemon juice, Solvent = water.
Gas Solutions:
- Air: A mixture of nitrogen, oxygen, and other gases.
- Carbonated Water: Solute = carbon dioxide, Solvent = water.
Solvation:
Solvation is the process by which a solvent dissolves a solute to form a solution. When the solvent is water, this process is called hydration, where the solute particles are surrounded by water molecules.
Representing Solution Composition
When describing solutions, we use concentration to indicate how much solute is dissolved in a given amount of solvent. The most common way to express concentration in chemistry is through molarity.
Molarity (M):
Molarity is defined as the number of moles of solute per liter of solution. The formula is:
Example: If 24 moles of HCl are dissolved in 2 liters of water, the molarity of the solution is:
Note: The numerator (moles of solute) only refers to the amount of solute, while the denominator (volume of solution) includes both the solute and solvent.
Other Ways to Represent Concentration:
While molarity is the most commonly used method, other methods include:
Mass Percent:
Molality (m):
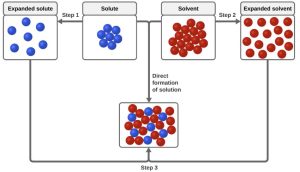
Forming a Solution:
Forming a solution involves three key steps:
- Expand the solute: Separate the solute into individual components.
- Expand the solvent: Break intermolecular forces in the solvent to make room for the solute.
- Solute-Solvent Interactions: Form new intermolecular forces between solute and solvent particles.

Diluting Solutions
Dilution involves decreasing the concentration of a solution by adding more solvent. The key equation for dilution is:
Where:
- and are the molarity and volume of the original solution.
- and are the molarity and volume of the diluted solution.
Why Does Dilution Work?
The number of moles of solute remains constant during dilution. By increasing the volume (adding solvent), the concentration decreases, following the equation
Example: If you have 100 mL of a 6 M NaCl solution and dilute it to 300 mL, what is the new molarity?
Using
The new molarity is 2 M.
Summary of Key Concepts:
- Solutions are homogeneous mixtures of solute and solvent.
- Molarity is the most common way to express concentration, calculated as moles of solute per liter of solution.
- Dilution involves decreasing concentration by adding solvent, using the equation
By understanding these key concepts, you’ll be ready to tackle solution-related problems on the AP Chemistry exam!