Understanding Electron Configurations and Orbital Diagrams
Electron configurations represent the arrangement of electrons in an atom’s orbitals. This concept is crucial for understanding the behavior of elements, as the way electrons are distributed determines chemical properties and bonding.
The Orbital Diagram Representation
In addition to the traditional electron configuration notation, we can visualize electron arrangements using orbital diagrams. These diagrams show how electrons fill different orbitals, using arrows to represent individual electrons.

Image Description: Each box represents an orbital, and each arrow represents a single electron. Electrons fill orbitals in order of increasing energy levels, following specific rules.
The Aufbau Principle states that electrons fill orbitals starting with the lowest energy level before moving to higher ones. In the diagram, you can see electrons filling orbitals in the order 1s, 2s, and then 2p, moving from the nucleus outward.
The Pauli Exclusion Principle is also represented in orbital diagrams by arrows pointing in opposite directions within an orbital. Each orbital can hold a maximum of two electrons, and they must have opposite spins. This rule ensures that no two electrons in the same orbital will have the same set of quantum numbers.
Hund’s Rule states that electrons will fill unoccupied orbitals first, before pairing up. This is because electrons tend to spread out as much as possible to minimize repulsion. The visual representation of Hund’s Rule is especially evident in p, d, and f orbitals, where multiple suborbitals are available:
Correct Example: Each electron fills an empty orbital before any orbitals receive a second electron. This approach results in a lower-energy configuration.
Incorrect Example: Pairing up electrons prematurely leads to unnecessary electron-electron repulsion.
Writing Electron Configurations: Example with Iron (Fe)
To understand electron configurations better, let’s look at element 26, iron (Fe).
Iron’s position in the periodic table tells us it includes electrons in the d block as part of its configuration. Here’s the complete electron configuration for iron:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶
Notice that the 4s orbital fills before the 3d. This is because the 4s orbital has a slightly lower energy than the 3d orbital when it is initially filled. However, during ionization or chemical bonding, the 4s electrons may be removed first.
To simplify electron configurations, we can use the noble gas shortcut. For iron, we use argon (Ar), the preceding noble gas:
[Ar] 4s² 3d⁶
This notation allows us to quickly reference the core electrons without rewriting the entire configuration from scratch.
Determining Core and Valence Electrons
Valence electrons are critical for predicting how an element will react. These are the outermost electrons involved in chemical bonding.
Consider the electron configuration of arsenic (As):
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3dⁱ⁰ 4p
Valence Electrons: Only the electrons in the highest energy level (n=4) are considered valence electrons. Here, arsenic has electrons in both the 4s and 4p orbitals.
Total Valence Electrons: 2 (from 4s) + 3 (from 4p) = 5 valence electrons.
Key Points to Remember
Aufbau Principle: Fill orbitals in order of increasing energy.
Pauli Exclusion Principle: No two electrons in the same orbital can have the same spin.
Hund’s Rule: Electrons will fill unoccupied orbitals before pairing up.
Valence Electrons: Only include the electrons in the outermost energy level—specifically in s and p orbitals—as they participate in bonding.
Understanding Atomic Structure, the Photoelectric Effect, and Photoelectron Spectroscopy
To understand the photoelectric effect and interpret photoelectron spectroscopy, it is essential to have a solid grasp of atomic structure. Recall that an atom is made up of three types of subatomic particles:
Protons: Located in the nucleus, with a positive charge (+1) and a mass of approximately 1 atomic mass unit (amu).
Neutrons: Located in the nucleus, without any charge (0), and a mass of approximately 1 amu.
Electrons: Found in orbitals around the nucleus, with a negative charge (-1) and an almost negligible mass (approximately 0 amu). In this study guide, our focus will be on the movement and behavior of electrons.
Electron Configuration Overview
Electrons are found in different energy levels, or shells, surrounding the nucleus. Within these shells, electrons are also distributed among subshells, represented by letters s, p, d, and f, with a maximum number of electrons per subshell:
s: Holds up to 2 electrons
p: Holds up to 6 electrons
d: Holds up to 10 electrons
f: Holds up to 14 electrons
For example, the electron configuration of boron (element 5 on the periodic table) is 1s² 2s² 2p¹.
The Quantum-Mechanical Model of the Atom
In the earlier sections, we discussed Bohr’s model of the atom, which depicted electrons as moving in fixed, circular orbits around the nucleus. Today, chemists prefer the quantum-mechanical model, which provides a more accurate representation of electron behavior.
Rather than precisely defining where electrons exist, the quantum-mechanical model uses probability to describe the regions where electrons are most likely to be found. These regions are called orbitals.
A key principle in this model is Heisenberg’s Uncertainty Principle, which states that it is impossible to simultaneously know both the exact position and momentum of an electron. In other words, the quantum-mechanical model tells us that electron behavior is inherently uncertain, and electrons behave as both particles and waves.
The Properties of Light 🌈
Before diving into the behavior of electrons, it’s important to understand the properties of light, as they relate closely to how electrons behave. One fascinating characteristic of both light and electrons is their wave-particle duality — they can exist as both a particle and a wave at the same time.
Light as a Particle (Photon)
Light can be understood as being made up of discrete packets of energy called photons. Each photon has a specific energy, as first proposed by Albert Einstein. The energy of a photon is directly related to its frequency (ν).
Frequency (v) refers to the number of wave cycles that pass a point in space per second. In simple terms, it’s like counting the number of ripples that pass in a second if you drop a pebble in water.
Higher frequency means higher energy for light, while lower frequency means lower energy.
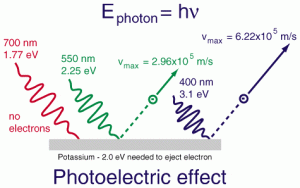
The Photoelectric Effect
The photoelectric effect is a phenomenon in which electrons are ejected from a metal surface when it is struck by light of sufficient energy. To put it simply, light hits the metal, and if the light’s energy is high enough, it “knocks” electrons out of the metal surface. These ejected electrons are called photoelectrons.
When light with low frequency (low energy) hits a metal, the metal absorbs the light, but no electrons are emitted. The light’s energy isn’t enough to overcome the force holding the electrons in place.
When light with high enough frequency hits the metal, electrons are emitted. This happens only when the energy of the incoming photons reaches or exceeds a specific energy threshold, known as the work function of the metal.

The photoelectric effect illustrates the particle-like nature of light and was fundamental in establishing quantum mechanics. This concept will be covered in greater detail in Unit 3 of the AP Chemistry curriculum.