What is an Ionic Bond: Everything You Need to Know
Have you ever wondered what holds salt together or how the energy in batteries is stored? The secret lies in a fundamental chemical process known as an ionic bond. In this comprehensive guide, we’ll answer the question what is an ionic bond by exploring its definition, history, mechanisms, properties, and real-world applications. Whether you’re a student grappling with basic chemistry concepts, a science enthusiast eager to deepen your understanding, or a professional looking for a refresher, this post will equip you with everything you need to know about ionic bonds.
Table of Contents
- Introduction: The Hidden Power of Ionic Bonds
- Defining Ionic Bonds: What Is an Ionic Bond?
- Historical and Contextual Background
- In-Depth Exploration: How Ionic Bonds Work
- Real-World Examples and Case Studies
- Importance, Applications, and Benefits of Ionic Bonds
- Common Misconceptions and FAQs
- Modern Relevance and Current Trends
- Conclusion: Embracing the Role of Ionic Bonds
- Additional Resources and Further Reading
1. Introduction: The Hidden Power of Ionic Bonds
Imagine the sparkle of a crystal, the reliable performance of your smartphone battery, or the taste of the salt sprinkled on your meal. What do these seemingly disparate things have in common? They all owe their properties to ionic bonds. These bonds are the invisible forces that hold together the compounds forming the backbone of many substances around us.
Did you know?
Lightning can be seen as nature’s display of ionization in the atmosphere, while everyday table salt is a prime example of an ionic compound formed through ionic bonding. These charged interactions are not just confined to dramatic natural phenomena; they are at work in our kitchens, laboratories, and industries.
In this post, we will cover:
- A clear and straightforward definition of what is an ionic bond.
- The historical evolution and key milestones in our understanding of ionic bonds.
- An in-depth exploration of how ionic bonds form, their properties, and how they influence the structure of matter.
- Real-world examples and case studies that illustrate the role of ionic bonds in everyday life, from common salt to advanced battery materials.
- The importance and applications of ionic bonds in various fields including industry, biology, and technology.
- Common misconceptions and frequently asked questions to clarify any doubts.
- Modern trends and emerging research related to ionic bonds.
By the end of this guide, you’ll not only know what is an ionic bond but also appreciate its profound impact on the natural world and human technology.
2. Defining Ionic Bonds: What Is an Ionic Bond?
A Straightforward Definition
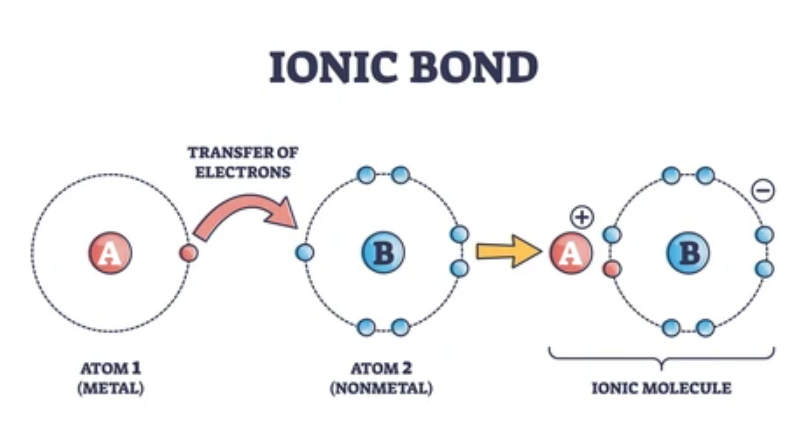
An ionic bond is a type of chemical bond that occurs when one atom donates one or more electrons to another atom, resulting in the formation of oppositely charged ions. These ions are then attracted to each other by strong electrostatic forces. In simple terms, what is an ionic bond? It is the attractive force that holds together a cation (a positively charged ion) and an anion (a negatively charged ion).
Essential Characteristics of Ionic Bonds
When exploring what is an ionic bond, it’s important to consider the following characteristics:
- Electron Transfer: Ionic bonds form as a result of complete transfer of electrons from one atom (usually a metal) to another (typically a nonmetal).
- Formation of Ions: This electron transfer creates ions: the donor atom becomes a cation and the recipient becomes an anion.
- Electrostatic Attraction: The oppositely charged ions attract each other strongly, forming a stable ionic compound.
- High Melting and Boiling Points: Due to the strong forces between ions, ionic compounds often have high melting and boiling points.
- Electrical Conductivity: In molten or dissolved states, ionic compounds can conduct electricity because the ions are free to move.
These defining properties answer the question what is an ionic bond and lay the groundwork for understanding its role in the formation of many common materials.
3. Historical and Contextual Background
Early Discoveries and Theoretical Foundations
The concept of ionic bonding evolved over centuries as scientists began to explore the nature of matter. Here are some key historical milestones:
- Early Observations:
In the 18th century, chemists observed that substances like salts could conduct electricity when dissolved in water. This phenomenon hinted at the presence of charged particles. - Faraday’s Experiments:
In the early 1800s, Michael Faraday conducted pioneering work on electrolysis, showing that electrical current could decompose chemical compounds into charged particles. His experiments laid the foundation for understanding ionic conduction. - Arrhenius’s Ion Theory:
The Swedish chemist Svante Arrhenius, in 1884, proposed that certain substances dissociate into ions when dissolved in water. Arrhenius’s theory was revolutionary—it explained why solutions of salts and acids could conduct electricity and marked the birth of modern ion theory.
Milestones in Understanding Ionic Bonds
- Development of the Periodic Table:
The organization of elements in the periodic table by Dmitri Mendeleev and others provided insights into how metals and nonmetals interact. This understanding was crucial for recognizing why metals tend to lose electrons and nonmetals tend to gain electrons. - Advances in Quantum Mechanics:
The 20th century brought significant advances in quantum mechanics, which allowed scientists to predict electron configurations and understand the nature of chemical bonding, including ionic bonds, at a fundamental level. - Technological Innovations:
Research into ionic compounds has driven technological advancements. For example, the development of high-performance batteries and new ceramic materials relies on the controlled formation of ionic bonds.
Anecdotes from the Past
One intriguing anecdote involves the discovery of table salt’s crystalline structure. Early crystallographers used X-ray diffraction to reveal the orderly lattice arrangement of sodium and chloride ions in salt—a discovery that not only confirmed the nature of ionic bonding but also advanced our understanding of crystal chemistry.
This historical context enriches our understanding of what is an ionic bond and illustrates its significance in both scientific progress and practical applications.
4. In-Depth Exploration: How Ionic Bonds Work
To truly understand what is an ionic bond, we need to delve into its formation, structure, and the forces that govern it. This section breaks down the key concepts with clarity and detail.
4.1 The Mechanism of Ionic Bond Formation
Electron Transfer Process
Ionic bonds form when there is a complete transfer of electrons from one atom to another:
- Metal to Nonmetal Transfer:
Metals, which have relatively few electrons in their outer shell, tend to lose electrons easily. Nonmetals, with higher electron affinities, tend to gain electrons. For example, in sodium chloride (NaCl), sodium (Na) loses one electron to become Na⁺, and chlorine (Cl) gains that electron to become Cl⁻. - Achieving Noble Gas Configuration:
The transfer of electrons allows both the metal and the nonmetal to achieve a stable electron configuration, often resembling that of the nearest noble gas. This drive for stability is the energetic motivation behind ionic bond formation.
The Role of Ionization Energy and Electron Affinity
Two key concepts determine whether an ionic bond will form:
- Ionization Energy:
The energy required to remove an electron from an atom. Metals typically have low ionization energies, making it easier for them to lose electrons. - Electron Affinity:
The energy released when an atom gains an electron. Nonmetals usually have high electron affinities, favoring the acquisition of electrons.
The difference between these energies helps explain what is an ionic bond at a quantitative level, predicting the likelihood of electron transfer and bond formation.
4.2 Key Characteristics of Ionic Bonds
Strong Electrostatic Forces
Once the ions are formed, they attract each other through strong electrostatic forces, which are governed by Coulomb’s Law:
- Coulomb’s Law:
The force of attraction (or repulsion) between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. - Lattice Energy:
The energy released when ions in the gas phase come together to form a solid ionic compound is known as lattice energy. High lattice energy is indicative of strong ionic bonds and contributes to the high melting and boiling points of ionic compounds.
Physical Properties of Ionic Compounds
The characteristics of ionic bonds impart several distinct properties to ionic compounds:
- High Melting and Boiling Points:
The strong attractions between ions require a significant amount of energy to break. - Brittleness:
Ionic compounds tend to be brittle because when a force shifts the ions, like-charged ions may come into contact and repel each other, causing the material to fracture. - Electrical Conductivity:
In their solid form, ionic compounds do not conduct electricity due to the fixed position of ions in the lattice. However, when melted or dissolved in water, the free movement of ions allows for electrical conductivity.
4.3 Ionic Compounds and Crystal Lattices
Formation of Crystal Lattices
Ionic compounds typically form crystalline structures:
- Lattice Structure:
The ions arrange themselves in a highly ordered, repeating pattern called a crystal lattice. The geometric arrangement maximizes the attractive forces and minimizes the repulsive forces between ions. - Examples:
Sodium chloride (NaCl) is one of the best-known examples, where each sodium ion is surrounded by six chloride ions and vice versa, forming a cubic lattice.
Impact on Material Properties
The structure of the ionic lattice is directly related to the physical properties of the compound:
- Hardness:
The rigid, interlocking lattice contributes to the hardness of many ionic compounds. - Solubility:
When ionic compounds dissolve in polar solvents like water, the ions are separated and stabilized by the solvent molecules. This solvation process is crucial in many biological and chemical systems.
4.4 Ionic Bonds in Aqueous Solutions
Dissociation and Conductivity
When ionic compounds dissolve in water, they dissociate into individual ions:
- Electrolytes:
Solutions containing free-moving ions are known as electrolytes. These solutions can conduct electricity, which is essential for processes such as nerve impulse transmission and muscle contraction. - Hydration:
Water molecules surround and stabilize the ions through hydration shells, which helps to keep the ions in solution.
pH and Ionic Strength
Ionic bonds play a significant role in determining the chemical properties of solutions:
- pH Levels:
The dissociation of acids and bases into ions directly influences the pH of a solution. - Ionic Strength:
The overall concentration of ions in a solution affects reaction rates, solubility, and other chemical equilibria.
Understanding the behavior of ionic bonds in solution is vital for both industrial processes and biological systems.
5. Real-World Examples and Case Studies
5.1 Table Salt (Sodium Chloride)
One of the most familiar examples of ionic bonding is found in table salt:
- Composition:
Sodium chloride (NaCl) is formed by the ionic bond between Na⁺ and Cl⁻. - Properties:
NaCl dissolves readily in water, forming a solution that conducts electricity due to the free movement of sodium and chloride ions. - Everyday Impact:
Besides its culinary uses, table salt is essential for maintaining fluid balance and nerve function in living organisms.
5.2 Calcium Oxide and Other Ionic Compounds
Calcium oxide (CaO) is another example that illustrates the strength and utility of ionic bonds:
- Formation:
CaO forms when calcium (a metal) loses two electrons to become Ca²⁺ and oxygen (a nonmetal) gains those electrons to become O²⁻. - Applications:
Calcium oxide, commonly known as quicklime, is used in the construction industry, in steelmaking, and in environmental applications like water treatment. - Properties:
The high lattice energy of CaO results in a compound with a high melting point and significant hardness.
5.3 Ionic Bonds in Biological Systems
Ionic bonds are not limited to inanimate matter—they also play a crucial role in biology:
- Nerve Signal Transmission:
The movement of ions such as sodium (Na⁺) and potassium (K⁺) across cell membranes is fundamental to generating and transmitting nerve impulses. - Muscle Contraction:
Calcium ions (Ca²⁺) are essential for muscle contraction and relaxation. - Enzyme Function:
Many enzymes require the presence of specific ions as cofactors to function properly, influencing metabolism and cellular processes.
These examples demonstrate the practical significance of ionic bonds and answer the question what is an ionic bond by showing its role in both everyday materials and complex biological systems.
6. Importance, Applications, and Benefits of Ionic Bonds
Understanding what is an ionic bond has far-reaching implications in various fields, including everyday life, industry, and science.
6.1 Ionic Bonds in Everyday Life
- Nutrition and Health:
Dietary electrolytes such as sodium, potassium, and calcium are crucial for maintaining bodily functions, including nerve conduction, muscle contraction, and fluid balance. - Household Products:
Many cleaning agents and personal care products contain ionic compounds that help to remove dirt and oil effectively. - Culinary Uses:
Table salt, an ionic compound, is essential not only for flavoring food but also for preserving it.
6.2 Industrial and Technological Applications
- Battery Technology:
Ionic compounds are at the heart of battery chemistry. For example, the movement of lithium ions in lithium-ion batteries is crucial for storing and releasing electrical energy in portable electronics and electric vehicles. - Water Treatment:
Ion exchange processes are used in water purification systems to remove contaminants and improve water quality. - Construction Materials:
Many building materials, such as cement and ceramics, rely on ionic bonds for their structural integrity.
6.3 Contributions to Science and Research
- Chemical Reactions:
The formation and breaking of ionic bonds are central to many chemical reactions, and understanding these processes helps scientists design new materials and pharmaceuticals. - Biochemical Processes:
Ionic bonds govern critical biological interactions, from cellular signaling to enzyme activity, offering insights into disease mechanisms and potential therapeutic targets. - Material Science:
Research into the properties of ionic compounds informs the development of novel materials with unique electrical, thermal, and mechanical properties.
By understanding what is an ionic bond, researchers and professionals can innovate in fields ranging from energy storage to healthcare, making this knowledge a cornerstone of modern science and technology.
7. Common Misconceptions and FAQs
Even among those familiar with basic chemistry, several misconceptions about ionic bonds persist. Let’s clarify some of these points and answer frequently asked questions.
7.1 Common Misconceptions
Misconception 1: Ionic Bonds Are Weak and Easily Broken.
Reality:
Ionic bonds are generally very strong due to the intense electrostatic attraction between oppositely charged ions. Their high lattice energies contribute to high melting and boiling points.Misconception 2: Ionic Bonds Only Occur in Salts.
Reality:
While table salt is a classic example, ionic bonds form the basis of many compounds, including metal oxides, sulfates, and even some complex minerals.Misconception 3: Ionic Compounds Conduct Electricity in All States.
Reality:
In their solid state, ionic compounds do not conduct electricity because the ions are fixed in the crystal lattice. However, they conduct when melted or dissolved in water.
7.2 Frequently Asked Questions (FAQs)
Q1: What is an ionic bond?
A1: An ionic bond is a chemical bond formed through the complete transfer of electrons from one atom to another, resulting in the formation of oppositely charged ions that attract each other.
Q2: How does an ionic bond differ from a covalent bond?
A2: In an ionic bond, electrons are transferred from one atom to another, while in a covalent bond, electrons are shared between atoms.
Q3: Why do ionic compounds have high melting and boiling points?
A3: The strong electrostatic forces between the ions in a crystal lattice require a significant amount of energy to overcome, resulting in high melting and boiling points.
Q4: Can ionic compounds conduct electricity?
A4: Yes, but only when they are in a molten state or dissolved in water, where the ions are free to move and carry electrical charge.
Q5: How are ionic bonds relevant in biological systems?
A5: Ionic bonds are crucial for maintaining the function of enzymes, the transmission of nerve impulses, and the regulation of fluid balance, among other biological processes.
8. Modern Relevance and Current Trends
The study and application of ionic bonds continue to evolve as new technologies and scientific methods emerge.
8.1 Emerging Technologies and Ionic Bonds
- Next-Generation Batteries:
Research into alternative battery chemistries, such as sodium-ion and solid-state batteries, relies heavily on understanding the movement of ions and the stability of ionic bonds within the electrode materials. - Ionic Liquids:
These are salts that are liquid at room temperature and offer unique properties like low volatility and high thermal stability. Ionic liquids are being explored as green solvents, in catalysis, and for advanced energy storage systems. - Nanotechnology and Material Synthesis:
Precise control over ionic bonding is paving the way for the creation of novel nanomaterials with tailored electrical and mechanical properties. These materials have applications in electronics, sensors, and structural components.
8.2 Environmental and Health Considerations
- Water Purification and Desalination:
Ion exchange and membrane technologies that rely on ionic interactions are key to developing efficient water purification systems and desalination processes, which are critical in addressing global water scarcity. - Atmospheric Studies:
Ions in the atmosphere, generated by natural processes or pollution, play a role in weather patterns and climate. Research into these processes is essential for environmental monitoring and developing strategies to mitigate pollution. - Medical Diagnostics:
Ion-selective electrodes and other diagnostic tools that measure the concentration of ions in biological fluids are essential for monitoring patient health and diagnosing conditions related to electrolyte imbalances.
As technology advances, the fundamental question what is an ionic bond remains as relevant as ever. New applications and research continue to expand our understanding of ionic bonds, ensuring that they remain a dynamic area of study and innovation.
9. Conclusion: Embracing the Role of Ionic Bonds
In this deep dive into what is an ionic bond, we have explored the nature of ionic bonding from its basic definition to its far-reaching implications in science and technology. Here’s a brief recap of the key points:
Definition:
An ionic bond is formed by the transfer of electrons from one atom to another, resulting in the formation of oppositely charged ions held together by strong electrostatic forces.Mechanism and Characteristics:
Ionic bonds are characterized by high lattice energies, high melting and boiling points, and the ability to conduct electricity when dissolved or melted. The formation of a crystal lattice gives rise to the unique properties of ionic compounds.Historical Evolution:
From early observations of electrical conductivity in solutions to groundbreaking experiments by Faraday and the development of ion theory by Arrhenius, our understanding of ionic bonds has evolved significantly over the centuries.Real-World Impact:
Ionic bonds are fundamental to everyday substances like table salt, crucial for biological functions such as nerve transmission and muscle contraction, and pivotal in advanced technologies including battery design and water purification.Modern Trends:
Research into ionic bonds continues to drive innovation in energy storage, nanotechnology, and environmental science, demonstrating that while the concept is ancient, its applications are modern and ever-evolving.
Understanding what is an ionic bond not only deepens our knowledge of chemistry but also highlights the interconnectedness of natural phenomena and technological advancements. As we continue to explore the microscopic forces that govern matter, ionic bonds remain a cornerstone of both fundamental science and practical application.
Call-to-Action
Share Your Thoughts:
How have you encountered ionic bonds in your daily life or work? Whether through the salt on your table, the battery in your phone, or the intricate processes of biological systems, we’d love to hear your experiences. Leave a comment below and join the conversation!Further Exploration:
If this guide has sparked your interest, explore our other posts on chemical bonding, atomic structure, and modern materials science. Deepening your understanding of these concepts can enhance your knowledge in both academic and practical settings.Stay Informed:
Subscribe to our newsletter for regular updates on the latest scientific discoveries, technological innovations, and in-depth articles on topics that matter. Together, we can continue to unravel the mysteries of the natural world, one bond at a time.
10. Additional Resources and Further Reading
For those looking to delve deeper into the fascinating world of ionic bonds and related topics, here are some reputable resources and further reading recommendations:
Wikipedia – Ionic Bond:
Learn more about ionic bonds and their properties.Khan Academy – Chemical Bonds:
Explore interactive lessons and videos on ionic bonds and other types of chemical bonding.American Chemical Society (ACS):
Read articles and research papers on ionic compounds and chemical bonding.Books:
- “Chemistry: The Central Science” by Brown, LeMay, Bursten, and Murphy – A comprehensive resource covering fundamental concepts in chemistry, including ionic bonding.
- “Inorganic Chemistry” by Shriver & Atkins – An in-depth exploration of chemical bonding and inorganic compounds.
Online Courses:
Platforms like Coursera, edX, and Udemy offer courses in chemistry and materials science that provide a deeper understanding of ionic bonding and its applications.
Final Thoughts
Ionic bonds are the invisible architects of the material world. They hold together the compounds that make up everyday substances, drive technological advancements in energy storage and electronics, and play an essential role in the complex chemistry of life. By understanding what is an ionic bond, we not only unlock the secrets of matter but also gain insight into the fundamental forces that shape our universe.
As we continue to push the boundaries of scientific knowledge and technological innovation, the study of ionic bonds remains as vital as ever. Embrace the power of ionic bonds, explore their myriad applications, and let this knowledge inspire you to see the world through the lens of chemistry and science.
If you found this guide helpful, please share it with friends, colleagues, and anyone curious about the wonders of chemistry. Leave your comments, ask questions, and let us know what topics you’d like us to explore next. Together, we can continue to learn, innovate, and appreciate the incredible science that underpins our everyday lives.
Happy exploring and learning!