“What is Physical Change” Everything You Need to Know: Exploring the Transformations Around Us
Have you ever noticed how ice melts into water on a hot day, or how a piece of chalk crumbles when dropped? These everyday observations lead us to an important scientific concept: physical change. But what is physical change? In this comprehensive guide, we will delve into the definition, characteristics, historical context, and modern applications of physical change. Whether you’re a student, teacher, or lifelong learner, this article will provide you with a deep understanding of how physical changes shape the world around us.
Introduction: Uncovering the Transformations in Our World
Imagine watching a caterpillar transform into a butterfly. While that metamorphosis is a biological change, there are countless examples of physical changes happening every day all around us. From the melting of ice to the bending of metal, physical changes are a fundamental part of the natural world.
Did you know that physical changes are not just limited to dramatic transformations but also include everyday events like dissolving sugar in your tea or shredding paper? In this article, we will explore:
- A clear and straightforward definition of physical change
- The historical development and understanding of physical changes
- Key characteristics that distinguish physical changes from chemical changes
- Real-world examples and case studies illustrating physical changes
- The importance of physical changes in science, industry, and daily life
- Common misconceptions and frequently asked questions about physical changes
- Modern relevance and current trends in the study of physical changes
By the end of this post, you will have a thorough understanding of what is physical change, why it matters, and how it impacts everything from natural phenomena to technological innovations. Let’s embark on this journey of discovery together.
What is Physical Change? A Straightforward Definition
Physical change refers to a process in which a substance undergoes a change in form, appearance, or state without altering its chemical composition. In other words, the molecules that make up the substance remain the same, even though the physical form might look different.
Essential Characteristics of Physical Change
Reversibility:
Many physical changes are reversible. For example, water can freeze into ice and then melt back into water without any change in its molecular structure.No New Substances Formed:
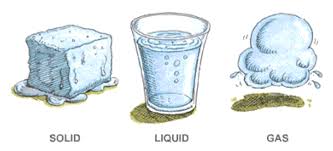
Unlike chemical changes, physical changes do not create new substances. The original material retains its identity, whether it’s changing state (solid, liquid, gas) or altering its shape or size.Changes in Physical Properties:
Physical changes can affect properties such as shape, size, phase (solid, liquid, gas), texture, and color. However, the substance’s chemical composition remains intact.Energy Involvement:
While physical changes can involve energy transfer (like melting ice absorbing heat), this energy does not break or form chemical bonds.
Understanding these characteristics provides a strong foundation for exploring how physical changes occur and why they are significant in both science and everyday life.
Historical and Contextual Background
The study of physical changes has been an essential part of science for centuries. Early scientists and philosophers were fascinated by natural phenomena, and their observations laid the groundwork for modern physics and chemistry.
Early Observations and Philosophical Roots
Ancient Philosophy and Natural Phenomena:
Ancient Greek philosophers like Aristotle observed that substances could change forms. Aristotle’s ideas about matter and change, though not fully accurate by modern standards, sparked early interest in understanding how and why substances transformed.Medieval Contributions:
During the Middle Ages, scholars began to document observations about physical changes, such as the melting of metals and the solidification of molten materials. These early studies were crucial in developing early theories of matter.
Scientific Milestones
The Scientific Revolution:
The 16th and 17th centuries marked a period of rapid advancement in scientific understanding. Pioneers like Galileo Galilei and Sir Isaac Newton laid the foundations for classical mechanics, which would later help scientists understand the forces and energy changes involved in physical transformations.Development of Thermodynamics:
In the 19th century, the study of thermodynamics provided a detailed understanding of energy changes during physical processes. The concepts of heat, work, and energy conservation helped scientists explain why physical changes like melting and evaporation occur.Modern Physics and Material Science:
The 20th century saw significant advances in material science and physical chemistry. Researchers developed sophisticated tools to observe and measure physical changes at the microscopic level, further deepening our understanding of these phenomena.
Notable Historical Anecdotes
The Melting of Ice:
One of the simplest examples of physical change is the melting of ice into water. Early experiments in thermodynamics used ice melting as a model to study heat transfer and phase transitions.Phase Diagrams:
The development of phase diagrams in the 19th century allowed scientists to map out the conditions under which substances change states (e.g., solid, liquid, gas). These diagrams remain a crucial tool in both education and industrial applications today.
By tracing the historical evolution of our understanding of physical change, we can appreciate how scientific inquiry has transformed simple observations into a comprehensive framework that underpins much of modern science.
In-Depth Exploration: Understanding the Mechanisms of Physical Change
To fully grasp what is physical change, it is essential to explore the underlying mechanisms and key factors that drive these transformations. This section will break down the processes involved in physical changes, using clear examples and detailed explanations.
1. Phase Changes: Transitioning Between States of Matter
Phase changes are perhaps the most common examples of physical change. They involve the transformation of a substance from one state of matter to another without changing its chemical identity.
a. Melting and Freezing
Melting:
Melting is the process in which a solid absorbs heat and transitions into a liquid. For instance, when ice absorbs heat, it melts into water. This process is endothermic, meaning it requires the absorption of energy.Freezing:
Freezing is the reverse process where a liquid loses heat and becomes a solid. When water loses heat, it freezes into ice. This process is exothermic, releasing energy into the surroundings.Real-World Example:
Consider the process of making ice cubes in a freezer. Water placed in an ice tray undergoes freezing—a physical change—while its chemical composition (H₂O) remains unchanged.
b. Evaporation and Condensation
Evaporation:
Evaporation occurs when a liquid absorbs enough heat to change into a gas. This is why puddles dry up on a sunny day as water evaporates into the air.Condensation:
Conversely, condensation happens when a gas loses heat and turns back into a liquid. This is observed when water droplets form on the outside of a cold beverage glass.Real-World Example:
The water cycle is a natural phenomenon that relies on evaporation and condensation. Water from oceans, lakes, and rivers evaporates, forms clouds through condensation, and eventually precipitates back to Earth as rain or snow.
c. Sublimation and Deposition
Sublimation:
Sublimation is the process where a solid turns directly into a gas without passing through the liquid phase. Dry ice (solid CO₂) is a classic example, as it sublimates at room temperature.Deposition:
Deposition is the reverse of sublimation, where a gas transforms directly into a solid. Frost formation on a cold surface is an example of deposition.Real-World Example:
Sublimation is used in freeze-drying food, where water is removed from the food in a way that preserves its structure and nutritional value.
2. Changes in Shape and Form
Not all physical changes involve a change in state. Some involve changes in shape, size, or appearance while maintaining the same material composition.
a. Cutting, Bending, and Shaping
Mechanical Changes:
Physical processes such as cutting, bending, stretching, and crushing alter the form or shape of a substance without changing its chemical structure.Real-World Example:
When you cut a piece of paper, you change its shape and size, but the paper remains chemically the same.
b. Dissolving
Solvation Process:
Dissolving involves a solid, liquid, or gas dispersing into a solvent to form a solution. This is a physical change because no chemical bonds are broken; the solute simply disperses throughout the solvent.Real-World Example:
When you stir sugar into a cup of tea, the sugar dissolves. The sugar molecules spread out evenly in the tea, but their chemical structure remains unchanged.
c. Mixing
Physical Mixtures:
Mixing different substances together can result in a physical change if no chemical reaction occurs. The individual components remain distinct even if they are uniformly distributed.Real-World Example:
A salad is a physical mixture where each ingredient retains its own properties, despite being combined into one dish.
3. Energy Changes in Physical Processes
Although physical changes do not involve a change in chemical composition, they often require energy input or result in energy release.
a. Endothermic Processes
Energy Absorption:
Endothermic processes, such as melting and evaporation, absorb energy from their surroundings. This energy is used to overcome the forces holding the molecules in a particular state.Real-World Example:
Melting ice absorbs heat from the environment, which is why it feels cold to the touch.
b. Exothermic Processes
Energy Release:
Exothermic processes, such as freezing and condensation, release energy into the surroundings. This energy release often results in a temperature increase of the immediate environment.Real-World Example:
When water freezes, it releases latent heat, which can be observed in certain natural phenomena like the warming of the air around a freezing lake.
4. The Role of Pressure and Temperature
Pressure and temperature are two key factors that influence physical changes. Adjusting these parameters can dramatically alter the state and form of a substance.
a. Temperature
Impact on Kinetic Energy:
Temperature affects the kinetic energy of particles. As temperature increases, particles move faster, which can lead to changes in state, such as melting or evaporation.Real-World Example:
A metal rod expands when heated due to the increased movement of its atoms, demonstrating a physical change in its dimensions.
b. Pressure
Influence on Phase Transitions:
Pressure plays a crucial role in phase transitions. High pressure can force a gas to condense into a liquid, or even into a solid, as seen in the formation of diamonds from carbon under extreme conditions.Real-World Example:
Carbon dioxide can exist as a supercritical fluid under high pressure and temperature—a state that exhibits properties of both a gas and a liquid.
Importance, Applications, and Benefits of Understanding Physical Change
Understanding what is physical change is vital for multiple disciplines, from science and engineering to everyday problem-solving. Here’s why this concept is so important:
1. Fundamental to Science and Engineering
Basis for Material Science:
Physical changes are central to material science, where understanding the properties and behaviors of materials under different conditions is crucial for innovation and development.Industrial Applications:
Processes such as melting, freezing, and dissolving are essential in manufacturing and industrial processes. For example, the food industry relies on freeze-drying (a physical change process) to preserve food without altering its nutritional content.
2. Everyday Life and Practical Applications
Cooking and Food Preparation:
Many cooking techniques rely on physical changes. Boiling, freezing, and even cutting ingredients are all physical processes that alter the texture and form of food without changing its chemical makeup.Household Activities:
From dissolving detergent in water to shaping dough into bread, understanding physical change helps us comprehend and improve our daily tasks.
3. Educational Impact
Foundation for Learning:
Grasping physical change is a fundamental part of science education. It serves as a stepping stone to understanding more complex chemical reactions and physical phenomena.Encouraging Critical Thinking:
Studying physical changes encourages critical thinking and problem-solving skills by requiring students to analyze how and why changes occur without altering a substance’s identity.
4. Environmental and Technological Relevance
Climate Science:
Understanding phase changes, such as the melting of ice caps or the evaporation of water from oceans, is crucial in climate science and environmental studies.Innovative Technologies:
Advances in technology, such as the development of new materials and energy-efficient processes, often depend on manipulating physical changes under controlled conditions.
Addressing Common Misconceptions and FAQs about Physical Change
Despite being a fundamental concept, several misconceptions about physical change persist. Let’s address some of these and provide clear answers to frequently asked questions.
Common Misconceptions
Misconception 1: Physical Changes are Irreversible
Reality:
Many physical changes are reversible. For instance, water can freeze and then melt back into liquid form without any change in its chemical structure.Misconception 2: Physical Changes Always Involve a Change in Temperature
Reality:
While temperature often plays a role in physical changes, some changes—such as cutting or dissolving—can occur at constant temperatures.Misconception 3: Physical Changes are Less Significant than Chemical Changes
Reality:
Physical changes are crucial in many fields, including materials science, environmental science, and engineering. They form the basis of numerous everyday processes and industrial applications.
Frequently Asked Questions (FAQs)
Q: What exactly is physical change?
A:
Physical change is a transformation that alters the form or appearance of a substance without changing its chemical composition.Q: Can physical changes be reversed?
A:
Many physical changes are reversible. For example, water can transition from ice to liquid and back to ice repeatedly without any alteration in its chemical structure.Q: How can you distinguish between a physical change and a chemical change?
A:
In a physical change, the substance’s chemical makeup remains the same; only its physical form is altered. In contrast, a chemical change results in the formation of new substances with different properties.Q: Are processes like dissolving and mixing considered physical changes?
A:
Yes, dissolving and mixing are physical changes because they involve the dispersion or rearrangement of particles without altering the substance’s chemical structure.Q: Why is understanding physical change important?
A:
Understanding physical change is essential for scientific study, industrial applications, environmental monitoring, and everyday problem-solving, as it explains how substances behave under various conditions.
Modern Relevance and Current Trends in the Study of Physical Change
As our world becomes increasingly dependent on technology and innovation, the study of physical change remains as relevant as ever. Here are some modern trends and developments in this field:
1. Advancements in Material Science
Nanotechnology:
The manipulation of materials at the nanoscale often relies on controlled physical changes. Researchers are exploring how phase transitions and structural changes can be harnessed to develop new nanomaterials with unique properties.Smart Materials:
Smart materials, which can change their properties in response to external stimuli (like temperature or pressure), are a direct application of controlled physical change. These materials are used in everything from medical devices to aerospace engineering.
2. Environmental and Climate Research
Cryospheric Studies:
The melting of glaciers and polar ice caps is a physical change with profound environmental implications. Understanding these processes is crucial for predicting climate change and developing strategies for environmental protection.Water Cycle Analysis:
Advances in satellite imaging and remote sensing technology allow scientists to monitor physical changes in water bodies, such as evaporation and condensation, providing critical data for climate models.
3. Industrial and Technological Innovations
Energy-Efficient Processes:
Industries are continually seeking ways to optimize processes such as melting, freezing, and crystallization to save energy and reduce costs. Innovations in these areas can lead to significant improvements in manufacturing efficiency.Recycling and Sustainability:
Physical changes are at the heart of recycling processes. Understanding how materials can be physically reformed or repurposed without chemical alteration is essential for developing sustainable recycling technologies.
4. Educational and Research Applications
Interactive Learning Tools:
Modern educational tools now incorporate simulations and interactive models to demonstrate physical changes, making complex processes accessible to students of all ages.Research on Phase Transitions:
Cutting-edge research continues to explore the fundamental physics behind phase transitions, with applications ranging from superconductivity to the development of new energy storage systems.
Conclusion: Embracing the World of Physical Change
In our extensive exploration of what is physical change, we have journeyed through its definition, historical evolution, underlying mechanisms, and modern-day applications. Here are the key takeaways:
Definition and Characteristics:
Physical change refers to alterations in a substance’s physical form or appearance without changing its chemical composition. These changes include phase transitions, changes in shape, dissolving, and mixing.Historical Context:
From ancient philosophical observations to modern scientific breakthroughs, our understanding of physical change has evolved dramatically, forming the backbone of many scientific principles and industrial processes.Mechanisms and Examples:
Whether it’s the melting of ice, the dissolution of sugar in tea, or the reshaping of materials through cutting and bending, physical changes are omnipresent in both nature and technology.Importance and Applications:
Physical change is fundamental to science, engineering, environmental studies, and everyday life. Recognizing and understanding these changes enhances our ability to innovate, solve problems, and appreciate the natural world.Modern Trends:
Advancements in material science, environmental monitoring, and industrial processes continue to rely on and expand our understanding of physical changes, ensuring their relevance in an ever-evolving world.
Call to Action
Now that you have a comprehensive understanding of what is physical change, we encourage you to:
- Reflect: Think about the physical changes you observe in your daily life, from the simple act of ice melting to the more complex industrial processes.
- Explore Further: Delve deeper into related topics such as chemical changes, material science, and thermodynamics to broaden your scientific knowledge.
- Engage: Share your thoughts or questions in the comments below. What physical changes have you noticed recently, and how do you think they impact your world?
- Share: If you found this article informative, please share it with friends, educators, or anyone interested in the fascinating world of science and everyday transformations.
By embracing the concept of physical change, we not only enhance our understanding of the world but also empower ourselves to appreciate the intricate and beautiful processes that govern every aspect of our lives.
Additional Resources and References
For readers interested in further exploring what is physical change, here are some reputable sources and further reading materials:
Books and Academic Texts:
- “Chemistry: The Central Science” by Theodore L. Brown et al. – A comprehensive textbook covering physical and chemical changes.
- “Concepts of Modern Physics” by Arthur Beiser – Provides an in-depth look at the principles behind phase transitions and energy changes.
- “Introduction to Materials Science for Engineers” by James F. Shackelford – Explores how physical changes affect material properties and industrial applications.
Online Educational Resources:
- Khan Academy – States of Matter – Educational videos and articles on phase changes and physical properties.
- MIT OpenCourseWare – Free course materials on thermodynamics and material science.
- ChemCollective – Interactive simulations and problem-solving exercises related to physical and chemical changes.
Research Journals and Articles:
- Journal of Physical Chemistry – Explore cutting-edge research on physical changes at the molecular level.
- Nature Materials – Research articles on the latest advancements in material science and physical changes.
Workshops and Online Courses:
- Platforms such as Coursera, edX, and Udemy offer courses in thermodynamics, material science, and physics that cover physical changes in depth.
Final Thoughts
Physical changes are all around us—from the ice in your freezer to the steam rising from a hot cup of coffee. Understanding what is physical change enriches our knowledge of the natural world and empowers us to innovate and solve practical problems. This concept, which underpins many scientific and industrial processes, offers a window into the dynamic interplay between energy, matter, and the environment.
Thank you for joining us on this in-depth exploration of physical change. We hope this article has deepened your understanding and sparked your curiosity to further explore the wonders of science. If you enjoyed this post, please share it, leave your feedback, or ask your questions in the comments below. Let’s continue to celebrate the fascinating processes that shape our world, one physical change at a time.