“What Is Mass Number: Everything You Need to Know”
Mass number is one of the most fundamental concepts in chemistry and physics, yet it often remains shrouded in technical jargon. Have you ever wondered why scientists stress that Carbon-12 has a mass number of 12, or how the sum of protons and neutrons defines the identity of an element? In this comprehensive guide, we’ll demystify the concept of mass number by breaking down its definition, history, and wide-ranging applications—from nuclear energy and astrophysics to everyday chemistry. Whether you’re a student, an educator, or simply a curious mind, understanding what is mass number is essential for grasping the building blocks of matter and the processes that shape our universe.
Introduction
Imagine trying to understand the structure of an atom without knowing how many protons and neutrons it contains. Without mass number, much of modern science—from the design of nuclear reactors to the dating of ancient artifacts—would be impossible. In fact, the mass number of an element is so crucial that it not only defines the element’s identity but also plays a key role in determining its chemical and physical behavior.
Did you know that the mass number of Carbon-12 is exactly 12, a value so precise that it forms the basis for the atomic mass unit? This precision underpins everything from the periodic table to the models of stellar evolution. In this article, we will cover:
- A clear and concise definition of mass number.
- The essential characteristics and properties that define mass number.
- Historical and contextual background on the evolution of our understanding of mass number.
- An in-depth exploration of the key aspects and categories of mass number, complete with real-world examples and case studies.
- The significance, applications, and benefits of mass number in various fields such as science, technology, and everyday life.
- Common misconceptions and frequently asked questions to help clarify any doubts.
- Modern relevance and current trends, including recent research and debates related to mass number.
- A conclusion that summarizes the key points and reinforces the importance of understanding mass number, along with a call-to-action for further reading and engagement.
By the end of this guide, you’ll have a deep and nuanced understanding of what is mass number, its role in modern science, and how it impacts both theoretical research and practical applications.
What Is Mass Number? A Straightforward Definition
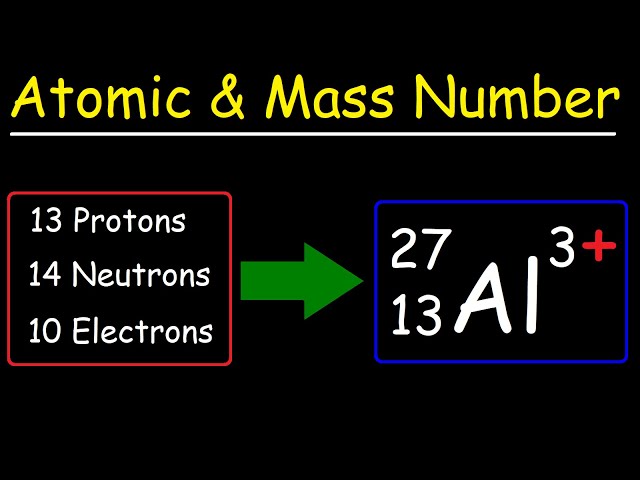
Mass number is defined as the total number of protons and neutrons in the nucleus of an atom. It is a whole number that gives us a measure of the overall mass of the atom (excluding electrons, whose mass is negligible compared to that of protons and neutrons). In mathematical terms:
Mass Number (A)=Number of Protons (Z)+Number of Neutrons (N)
Essential Characteristics of Mass Number
When we ask what is mass number, it’s important to consider several key properties:
- Integral Value: Mass number is always a whole number since it represents a count of particles.
- Nuclear Composition: It provides a direct measure of the nucleons (protons and neutrons) in the nucleus.
- Isotopic Differentiation: Isotopes of the same element have the same number of protons but different mass numbers due to varying numbers of neutrons.
- Stability Indicator: The mass number can be an indicator of nuclear stability; certain mass numbers correspond to more stable nuclei.
- Distinct from Atomic Mass: While atomic mass is a weighted average that takes into account the relative abundance of isotopes, mass number is a simple sum of nucleons.
In essence, what is mass number? It is a fundamental descriptor of an atom’s nucleus, encapsulating its basic makeup and playing a crucial role in both chemistry and nuclear physics.
Historical and Contextual Background
Early Concepts and Ancient Beginnings
Philosophical Origins
- Pre-Scientific Era: Ancient civilizations, such as the Greeks, pondered the nature of matter without the modern concept of atoms. Early ideas about “substance” and “essence” set the stage for later scientific inquiry.
- Empirical Observations: Early alchemists and philosophers observed that substances behaved differently, hinting at an underlying structure that would later be quantified.
The Birth of Atomic Theory
- John Dalton’s Atomic Theory (Early 1800s): Dalton’s work marked a turning point by proposing that elements are composed of atoms that differ in mass. Although his theory did not explicitly use the term “mass number,” it laid the foundation for understanding atomic composition.
- Isotopes and the Refinement of Mass Concepts: The discovery of isotopes in the early 20th century by Frederick Soddy further refined the concept of mass. Scientists began to understand that atoms of the same element could have different mass numbers due to variations in neutron count.
The Scientific Revolution and Modern Physics
Newton and the Quantification of Matter
- Classical Mechanics: Isaac Newton’s work in the 17th century introduced a systematic way to measure and calculate forces, including the gravitational forces that depend on mass. While Newton’s laws focused on the effects of mass, later scientists would build on his work to understand its origins.
Einstein’s Mass-Energy Equivalence
- Revolutionary Insight: In 1905, Albert Einstein’s equation provided a groundbreaking understanding that mass and energy are interchangeable. This revelation has had profound implications for both theoretical physics and practical applications like nuclear energy.
- Impact on Modern Science: Einstein’s work emphasized that mass is not merely a static property but is deeply connected to the fundamental forces and energy dynamics of the universe.
Notable Historical Anecdotes
- Dalton’s Experiments: John Dalton’s careful measurements and his proposal of atomic theory helped establish that elements have characteristic masses—a precursor to the concept of mass number.
- Discovery of Isotopes: The identification of isotopes in elements such as uranium and carbon illustrated that not all atoms of an element are identical, leading to the refinement of mass measurements and the concept of mass number.
- Einstein’s “Miracle Year”: In 1905, Einstein’s annus mirabilis produced several papers that would forever alter our understanding of mass, energy, and the very fabric of space-time.
In-Depth Exploration: Key Aspects and Categories of Mass Number
1. The Composition of an Atom
- Atomic Nucleus: The nucleus of an atom contains protons and neutrons. The mass number is simply the sum of these two types of particles.
- Isotopic Variations: Different isotopes of the same element have different mass numbers. For example, Carbon-12 and Carbon-14 are both isotopes of carbon but have mass numbers of 12 and 14, respectively.
2. Inertial and Gravitational Mass: The Role of Mass Number
- Inertial Mass: Mass number directly contributes to an atom’s inertial mass, which is a measure of its resistance to acceleration.
- Gravitational Mass: The mass number also influences gravitational mass, determining how strongly an atom interacts with gravitational fields.
- Equivalence Principle: The equivalence of inertial and gravitational mass is a cornerstone of classical and modern physics.
3. Mass Number and Nuclear Reactions
- Nuclear Stability: The mass number of a nucleus is a critical factor in determining its stability. Certain mass numbers correspond to stable configurations, while others may be prone to radioactive decay.
- Fission and Fusion: In nuclear reactions, such as fission (splitting heavy nuclei) and fusion (combining light nuclei), the mass number plays a key role in energy release. Even a small change in mass number can lead to a significant release of energy, as described by Einstein’s equation .
4. Measurement and Determination of Mass Number
- Mass Spectrometry: Modern techniques, such as mass spectrometry, allow scientists to measure the mass number of isotopes with incredible precision. This technology is essential for fields ranging from chemistry to astrophysics.
- Historical Methods: Early scientists used chemical reactions and stoichiometry to determine the mass numbers of elements, paving the way for more advanced techniques.
5. Real-World Examples and Case Studies
Case Study 1: Carbon Isotopes in Archaeology
- Carbon-14 Dating: Carbon-14, an isotope with a mass number of 14, is used in radiocarbon dating to determine the age of archaeological artifacts. By measuring the remaining Carbon-14 in organic materials, scientists can estimate the time elapsed since the organism’s death.
- Impact: This method has revolutionized archaeology, allowing for more accurate dating of ancient civilizations and artifacts.
Case Study 2: Uranium Isotopes in Nuclear Energy
- Uranium-235 vs. Uranium-238: Uranium has several isotopes, with Uranium-235 (mass number 235) being fissile and used as fuel in nuclear reactors, while Uranium-238 (mass number 238) is more abundant but not fissile.
- Impact: The difference in mass number between these isotopes is critical for nuclear energy production, affecting reactor design, fuel efficiency, and safety protocols.
Case Study 3: Stellar Nucleosynthesis
- Formation of Elements in Stars: The process of stellar nucleosynthesis involves the fusion of light nuclei to form heavier elements. The mass number of the resulting nuclei determines the energy released in these reactions, which in turn affects the lifecycle of stars.
- Impact: Understanding mass number is crucial for astrophysicists studying the evolution of stars and the distribution of elements in the universe.
Importance, Applications, and Benefits of Understanding Mass Number
Understanding what is mass number is essential for a variety of reasons that span scientific research, practical applications, and everyday life.
1. Advancing Scientific Knowledge
- Foundational in Chemistry and Physics: Mass number is a key parameter in the periodic table and nuclear reactions. It allows scientists to understand atomic structure, chemical behavior, and nuclear stability.
- Innovation in Energy and Materials: Research in nuclear energy, materials science, and astrophysics relies on precise measurements of mass number to drive innovation and discovery.
2. Practical Applications in Industry and Technology
- Nuclear Energy Production: The mass number of isotopes is critical in the design and operation of nuclear reactors, influencing efficiency and safety.
- Medical Diagnostics and Treatments: Radioisotopes, which are defined by their mass number, are used in medical imaging (such as PET scans and MRI) and in targeted cancer therapies.
- Environmental and Geological Studies: Mass number plays a role in dating rocks, tracing environmental changes, and understanding the composition of natural resources.
3. Everyday Relevance and Economic Impact
- Consumer Products: From the materials used in electronics to the food we eat, mass number influences the properties and behavior of the substances around us.
- Economic Indicators: In industrial processes, precise knowledge of mass numbers ensures quality control and optimization of production, leading to cost savings and improved performance.
4. Educational and Research Benefits
- STEM Education: A solid grasp of mass number is fundamental for students in science, technology, engineering, and mathematics. It enhances critical thinking and problem-solving skills.
- Interdisciplinary Insights: The concept of mass number bridges chemistry, physics, geology, and astronomy, fostering a holistic understanding of how the universe operates.
Addressing Common Misconceptions and FAQs
Despite its importance, several misconceptions about what is mass number persist. Let’s address some of these misunderstandings:
Misconception 1: Mass Number Is the Same as Atomic Mass
- Clarification: Mass number is the sum of protons and neutrons in an atom’s nucleus and is always a whole number. Atomic mass, however, is a weighted average of all isotopes of an element, accounting for their natural abundance, and is expressed in atomic mass units (amu).
Misconception 2: All Isotopes of an Element Have the Same Mass Number
- Clarification: Isotopes of an element have the same number of protons but different numbers of neutrons, resulting in different mass numbers. For example, Carbon-12 and Carbon-14 are both isotopes of carbon, but their mass numbers are 12 and 14, respectively.
Misconception 3: Mass Number Changes with Chemical Reactions
- Clarification: Chemical reactions involve the rearrangement of electrons, not changes in the nucleus. Therefore, the mass number remains constant during chemical reactions.
Frequently Asked Questions (FAQs)
Q1: What is mass number in simple terms?
A1: Mass number is the total number of protons and neutrons in the nucleus of an atom. It is an integer value that represents the atomic composition in terms of nucleons.Q2: How does mass number differ from atomic mass?
A2: Mass number is a whole number that counts the nucleons in a specific isotope, whereas atomic mass is a calculated average of the masses of all isotopes of an element, weighted by their natural abundance.Q3: Why is mass number important in nuclear reactions?
A3: The mass number determines the balance of nucleons in a nucleus, which affects its stability and the energy released during nuclear reactions such as fission and fusion.Q4: How do scientists measure mass number?
A4: Mass number is determined by counting the protons and neutrons in an atom’s nucleus. Techniques like mass spectrometry can precisely measure the mass of isotopes, indirectly providing the mass number.Q5: Can mass number change over time?
A5: In chemical reactions, the mass number remains constant because the nucleus is not altered. However, in nuclear reactions (such as radioactive decay), the mass number can change as the nucleus transforms.
Modern Relevance and Current Trends
In the modern scientific and technological landscape, the concept of what is mass number continues to evolve, driving new research and applications.
1. Advances in Nuclear Physics and Quantum Mechanics
- Refined Measurements: Modern mass spectrometers and particle accelerators allow scientists to measure mass numbers with unprecedented accuracy, deepening our understanding of atomic structure.
- Higgs Boson and Beyond: Research into the origin of mass at the subatomic level, including studies related to the Higgs boson, continues to refine our understanding of how mass is generated.
2. Applications in Renewable Energy and Medical Technology
- Nuclear Energy: Accurate mass numbers are critical for designing efficient and safe nuclear reactors. Ongoing research aims to improve reactor technology and waste management.
- Medical Isotopes: The development of radioisotopes for medical diagnostics and treatment relies on precise knowledge of mass numbers, impacting areas such as cancer therapy and imaging technologies.
3. Environmental and Geological Research
- Radiometric Dating: Mass number is key in radiometric dating techniques, which help scientists determine the age of rocks, fossils, and archaeological artifacts.
- Climate Studies: Isotopic analysis, which depends on mass numbers, is used to reconstruct past climates and understand environmental changes over geological timescales.
4. Educational and Public Outreach Initiatives
- Interactive Learning: Digital tools and simulations help students visualize how mass number relates to atomic structure and nuclear reactions, making the concept more accessible.
- STEM Engagement: Educational programs that emphasize the importance of mass number foster curiosity and critical thinking in the next generation of scientists and engineers.
5. Future Directions and Research Frontiers
- Nanotechnology: As research pushes into the nanoscale, understanding the precise mass numbers of atoms and molecules is crucial for developing new materials and technologies.
- Astrophysics: In the study of stars and galaxies, mass number plays a role in understanding stellar nucleosynthesis and the evolution of cosmic structures.
The Practical Benefits of Understanding Mass Number
A deep understanding of what is mass number offers numerous practical benefits:
1. Enhancing Scientific Research and Innovation
- Accurate Modeling: Precise mass numbers are essential for creating accurate models in physics, chemistry, and engineering, leading to more reliable predictions and innovations.
- Breakthrough Discoveries: Research that depends on mass number, such as nuclear physics and quantum mechanics, drives breakthroughs that can lead to new technologies and scientific paradigms.
2. Improving Industrial Processes and Technology
- Quality Control: In industries such as pharmaceuticals, materials science, and nuclear energy, knowing the exact mass number of isotopes ensures consistency, safety, and efficiency in production.
- Energy Efficiency: Nuclear reactors rely on precise mass numbers to optimize reactions, improve energy output, and minimize waste.
3. Advancing Medical Diagnostics and Treatments
- Radiotherapy and Imaging: Medical applications of radioisotopes, defined by their mass numbers, have revolutionized diagnostics and treatment methods, resulting in improved patient outcomes.
- Personalized Medicine: Understanding the properties of isotopes based on their mass numbers can lead to tailored therapies that are more effective and have fewer side effects.
4. Promoting Educational Excellence and Lifelong Learning
- STEM Curriculum: A clear understanding of mass number is fundamental for students in science, technology, engineering, and mathematics, helping them build a solid foundation for advanced studies.
- Public Understanding of Science: Educating the public about basic scientific concepts like mass number promotes informed decision-making and enhances scientific literacy.
5. Supporting Environmental and Cultural Research
- Geochronology: Mass number is crucial in radiometric dating, which helps in understanding the history of the Earth and the evolution of life.
- Cultural Heritage: Archaeologists use mass number in dating artifacts, which in turn helps to preserve and understand cultural heritage.
Conclusion: Embracing the Significance of Mass Number
Our exploration of what is mass number has revealed that mass number is a fundamental property of matter that plays a critical role in numerous fields—from the structure of atoms and the mechanics of nuclear reactions to applications in medicine, industry, and environmental science. It is not merely a number; it is a key that unlocks a deeper understanding of the natural world.
Key Takeaways:
- Definition: Mass number is the sum of the number of protons and neutrons in an atom’s nucleus, serving as a basic measure of an atom’s total nucleons.
- Historical Evolution: From early atomic theories and Dalton’s work to Einstein’s mass-energy equivalence and modern quantum mechanics, our understanding of mass number has evolved significantly.
- Scientific and Practical Applications: Mass number is critical in nuclear physics, chemistry, astrophysics, medicine, and many industrial processes. It informs everything from energy production to radiometric dating.
- Modern Relevance: Ongoing advances in measurement technology, interdisciplinary research, and practical applications continue to highlight the importance of mass number in today’s world.
- Benefits: A clear grasp of mass number enhances scientific research, improves industrial efficiency, advances medical treatments, supports educational excellence, and deepens our understanding of both natural and cultural history.
As you reflect on this guide, consider how mass number underpins the science and technology that shape our world—from the energy that powers our cities to the materials that build our homes. Embracing what is mass number not only enriches our understanding of matter at the atomic level but also empowers us to drive innovation and make informed decisions across diverse fields.
Call to Action:
- Join the Conversation: Share your thoughts, experiences, or questions about mass number in the comments below. How has the concept of mass number influenced your studies or work?
- Share This Post: If you found this guide insightful, please share it on social media or with friends, colleagues, and anyone interested in the fundamental concepts of science.
- Keep Exploring: Continue your journey into the fascinating realms of physics and chemistry by exploring additional resources, enrolling in online courses, and following the latest research on atomic structure and nuclear physics.
Additional Resources
For further exploration of what is mass number and its applications, consider these reputable sources:
- Encyclopedia Britannica – Mass
- NASA – Mass, Gravity, and the Universe
- MIT OpenCourseWare – Classical Mechanics
- American Physical Society – Advances in Nuclear Physics
- Khan Academy – Introduction to Atomic Structure
Final Thoughts
Mass number is a foundational concept that unlocks our understanding of the atomic world, influencing everything from nuclear energy production to the dating of ancient artifacts. By understanding what is mass number, we gain insights into the very structure of matter and the forces that govern our universe. This knowledge not only fuels scientific discovery but also enhances our everyday lives by underpinning the technologies and processes that drive modern society.
Thank you for joining us on this in-depth exploration of mass number. Stay curious, keep learning, and let the wonders of atomic science inspire you to explore the fundamental building blocks of our world.