3.2 Enzyme Catalysis
Enzyme Catalysis: The Key to Life’s Complexity
Enzyme catalysis plays a vital role in the complex organization of living systems, allowing cells to perform essential functions efficiently. Whether it’s breaking down nutrients to generate energy, synthesizing macromolecules like DNA, RNA, and proteins, or transferring information between molecules, enzymes are at the heart of every major biological process. These specialized proteins act as catalysts, speeding up chemical reactions within cells, and enabling the complex tasks necessary to sustain life.
Maintaining a highly organized system requires constant energy input, which helps keep enzymes functioning and powers the reactions they catalyze. This efficiency allows living systems to perform the wide range of chemical reactions needed to sustain life while maintaining a steady state. Enzymes are like molecular machines that need energy to work properly—their activity often involves energy-consuming reactions like ATP hydrolysis, electron transfers, or proton movement.
The constant turnover of macromolecules is another crucial aspect of life. Proteins, nucleic acids, and other macromolecules are continuously synthesized, degraded, and recycled—a process largely dependent on enzyme action. This continuous exchange of materials helps maintain cellular balance, growth, and response to changing environments.
What Is Catalysis?
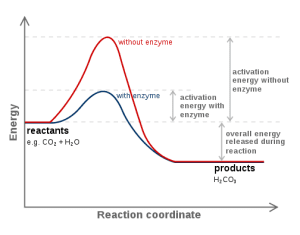
Catalysis refers to the process in which a catalyst speeds up a chemical reaction. Catalysts work by providing an easier pathway for reactants to transform into products, effectively lowering the activation energy (i.e., the amount of energy required for a reaction to proceed).

Catalysts achieve this by:Changing the positions of atoms in reactants, making it easier for them to form products.
Stabilizing intermediate products or transition states to make the reaction proceed smoothly.
Providing an alternative transition state that is more favorable for the reaction.

How Enzymes Catalyze Reactions
Enzymes are biomolecules that act as catalysts for chemical reactions in cells. They lower the activation energy of the reactions they catalyze, enabling the reaction to occur more quickly and efficiently. Enzymes are highly specific for their substrates (the reactants) due to their unique three-dimensional structure.
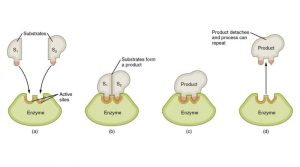
Enzyme-substrate recognition—where the enzyme binds to a specific substrate—forms an intermediate called the enzyme-substrate complex, which is more reactive than the substrate alone. Once the enzyme catalyzes the reaction, the substrate is converted into products that then leave the enzyme, freeing it to bind to another substrate molecule.
Enzymes can be reused in this way, making them highly efficient catalysts. The rate of enzyme-catalyzed reactions depends on several factors:
Substrate Concentration: Increasing substrate concentration can increase the reaction rate, up to a certain saturation point where the enzyme is fully occupied.
Temperature and pH: Enzymes function best at an optimal temperature and pH. Deviation from these conditions can lead to denaturation, where the enzyme loses its shape and becomes inactive.
Inhibitors and Activators:
Inhibitors are molecules that decrease enzyme activity. They may bind to the active site and block substrate binding or alter the enzyme’s shape.
Activators increase enzyme activity by stabilizing the enzyme-substrate complex.
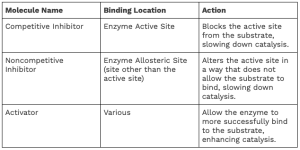
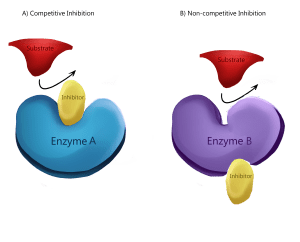
Competitive and Noncompetitive Inhibitors
Enzymes can be regulated by molecules that either inhibit or enhance their activity. Two main types of enzyme inhibitors are:


Competitive Inhibitors: These bind to the active site of the enzyme, directly blocking the substrate from binding. As a result, the enzyme’s function is inhibited. Competitive inhibition can often be overcome by increasing substrate concentration.
Noncompetitive Inhibitors: These bind to a site other than the active site (known as an allosteric site). Binding causes a change in enzyme shape, preventing the substrate from effectively binding, thereby reducing enzyme activity.
In addition to inhibitors, allosteric regulation is another mechanism by which enzyme activity can be modified. Molecules can bind to specific allosteric sites on the enzyme, causing it to either become more active or less active. These regulators allow cells to precisely control enzymatic reactions based on their needs.
Real-Life Application: The pH Experiment
Consider an experiment in which a scientist studies how pH affects the activity of an enzyme called “Protein X.” The scientist observes that the enzyme’s activity varies across different pH levels, with peak activity occurring at pH 8.0. Deviations from this optimal pH lead to decreased activity—a phenomenon linked to denaturation. Enzymes require optimal conditions to function effectively, and buffers help maintain these conditions in living cells by controlling pH fluctuations.
Key Takeaways
Enzymes are biological catalysts that speed up chemical reactions by lowering activation energy.
Catalysis is essential for the efficiency of biological reactions, enabling cells to maintain their highly complex organization.
Enzymes function best at specific temperatures and pH levels, and their activity can be regulated by activators, inhibitors, and allosteric modulators.
Both competitive and noncompetitive inhibitors can affect enzyme activity, offering mechanisms for controlling biological processes.
Enzyme catalysis is a cornerstone of cellular metabolism and the dynamic regulation of biological systems. Understanding how enzymes work and how they are regulated is crucial to mastering concepts in biochemistry and cellular biology.

Geometry Regents Score Calculator (2025 NY Exam Tool)
Geometry Regents Score Calculator Adjust the sliders below to calculate your potential Geometry Regents score Note: Raw scores are capped at 80 points for the