Tonicity & Osmoregulation: Understanding the Flow of Water in Cells
Water movement is fundamental to cellular life. The regulation of water across cellular membranes, known as osmosis, is vital for maintaining the stability and function of cells. In this blog post, we’ll explore key concepts such as tonicity and osmoregulation and how they contribute to maintaining cellular homeostasis. Let’s dive into what happens when a cell is placed in different solutions and how cells work to stay balanced.
Table of Contents
ToggleWhat is Tonicity?
Tonicity refers to the relative concentration of solutes in a solution compared to the inside of the cell. It determines the direction and extent of water movement across a semipermeable membrane. Depending on the relative solute concentration, the external environment of a cell can be classified as hypotonic, hypertonic, or isotonic. Each environment affects the cell differently:
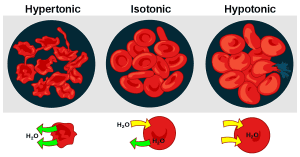
Hypotonic Solution: A solution with less solute than the inside of the cell. In this case, water will move into the cell, where there is a higher concentration of solute. This influx causes the cell to expand, and in extreme cases, it may even burst. Think of it as “HYPO = HIPPO” — the cell swells up like a hippo! 🦛
Hypertonic Solution: A solution with more solute outside the cell than inside. Water moves out of the cell to balance the concentration of solutes, causing the cell to shrink. Remember, “HYPER = Run outside!” 🏃♀️ Water leaves, and the cell shrivels.
Isotonic Solution: A solution where the concentration of solutes is equal inside and outside of the cell. Water moves in and out at equal rates, leading to no net change in cell shape. The cell remains stable and healthy. 💧
The movement of water in these scenarios is all about balancing solute concentrations inside and outside the cell. This movement of water is called osmosis.
Osmosis: The Key to Water Movement
Osmosis is the process by which water diffuses across a semipermeable membrane from an area of higher water concentration (lower solute concentration) to an area of lower water concentration (higher solute concentration). This helps cells maintain equilibrium, a state known as homeostasis.
In a hypotonic environment, water rushes into the cell, causing it to expand. In a hypertonic environment, water leaves the cell, causing it to shrink. In an isotonic environment, water moves in and out at the same rate, keeping the cell stable. The goal is always to balance solute levels on both sides of the membrane.
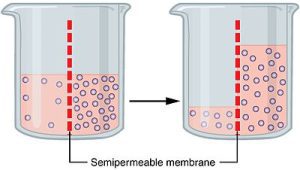
Osmoregulation: Balancing Water and Solutes
To survive and function properly, cells must carefully regulate their internal water levels — a process known as osmoregulation. Osmoregulation involves controlling water intake and loss to maintain a stable internal environment. Here’s how it works in different types of cells:
Animal Cells: Animal cells lack cell walls, making them more susceptible to changes in tonicity. In a hypotonic solution, an animal cell can take in so much water that it bursts. To avoid this, the cell relies on mechanisms like ion pumps to regulate water and solute movement. In a hypertonic solution, animal cells lose water and become shriveled.
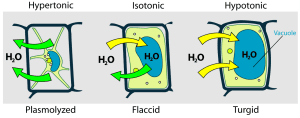
Plant Cells: Plant cells are better equipped to handle hypotonic environments because they have a rigid cell wall that prevents them from bursting. The cell wall maintains the cell’s shape, while the central vacuole stores excess water. In hypertonic environments, plant cells may experience plasmolysis, where the plasma membrane pulls away from the cell wall due to water loss, leading to wilting.
Water Potential: A Quantitative Measure
Water potential (ψ) is used to describe the direction in which water will move. Water moves from areas of high water potential to areas of low water potential. Water potential is influenced by solute potential (ψs) and pressure potential (ψp). In cells:
Adding solutes lowers water potential, making it more likely that water will move into that area.
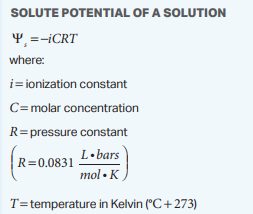
The solute potential equation is:
ψs = -iCRT
Where:
i is the ionization constant (e.g., 1 for sugar, 2 for NaCl).
C is the molar concentration.
R is the pressure constant (0.0831 L bar/mol K).
T is the temperature in Kelvin.

Water always moves to balance differences in water potential, moving from areas of higher potential to areas of lower potential to achieve equilibrium.
Why is Tonicity Important for Cells?
Understanding tonicity is essential for understanding how cells interact with their environment. In real-world applications, tonicity and osmoregulation are crucial for many processes, such as:
Medical IVs: The fluid in IV drips must be isotonic to prevent damage to patient cells.
Plant Watering: Overwatering or underwatering affects the tonicity of the plant’s environment, impacting growth.
Kidney Function: The kidneys rely on osmoregulation to manage water and solute levels in the body.
Cells need to regulate water movement constantly to prevent bursting or shrinking, ensuring they function optimally.
Conclusion
Tonicity and osmoregulation are vital processes that help cells maintain balance in varying environmental conditions. By understanding tonicity — whether hypotonic, hypertonic, or isotonic — and how osmoregulation supports cell stability, we can better appreciate how cells survive and thrive. Remember, water movement is a natural attempt to balance solute concentrations, helping cells maintain homeostasis.
Quick Quiz
How does osmosis contribute to homeostasis within cells?
Synthesizes proteins for cellular functions.
Facilitates communication between cells.
Produces energy for cellular processes.
Adjusts concentrations by moving water across membranes.
Answer: Adjusts concentrations by moving water across membranes.
Key Takeaways:
Tonicity refers to the relative concentration of solutes between two solutions.
Osmoregulation is the way cells manage water movement to maintain a stable environment.
Cells in hypotonic solutions swell, in hypertonic solutions shrink, and in isotonic solutions maintain balance.
Water potential helps predict the direction of water movement in a cell.
Recent Posts
- Laws of Indices – Number & Algebra – IB Mathematics AA HL
- Standard Form – Number & Algebra – IB Mathematics AA HL
- 9.2 Crafting an argument through stylistic choices like word choice and description
- 9.1 Strategically conceding, rebutting, or refuting information
- Unit 9 Overview: Developing a Complex Argument
- 8.4 Considering how style affects an argument
- 8.3 Considering how all choices made in an argument affect the audience
- 8.2 Considering how sentence development and word choice affect how the writer is perceived by an audience
- 8.1 Choosing comparisons based on an audience
- Unit 8 Overview: Stylistic Choices
- 7.4 Exploring how sentence development affects an argument
- Syllabus
- Study Guides for every class
- Short Notes
- 7.3 Examining how counterargument or alternative perspectives affect an argument
Choose Topic
- ACT (17)
- AP (20)
- AP Art and Design (5)
- AP Physics 1 (1)
- AQA (5)
- Artificial intelligence (AI) (2)
- Banking and Finance (6)
- Biology (13)
- Business Ideas (68)
- Calculator (72)
- ChatGPT (1)
- Chemistry (3)
- Colleges Rankings (48)
- Computer Science (4)
- Conversion Tools (136)
- Cosmetic Procedures (50)
- Cryptocurrency (49)
- Digital SAT (3)
- Edexcel (4)
- English (1)
- Environmental Science (2)
- Exam Updates (1)
- Finance (17)
- Fitness & Wellness (164)
- Free Learning Resources (209)
- GCSE (1)
- General Guides (40)
- Health (107)
- History and Social Sciences (152)
- IB (1)
- IGCSE (2)
- Image Converters (3)
- IMF (10)
- Math (39)
- Mental Health (58)
- News (8)
- OCR (3)
- Past Papers (463)
- Physics (5)
- SAT (39)
- Schools (3)
- Sciences (1)
- Short Notes (5)
- Study Guides (28)
- Syllabus (19)
- Tools (1)
- Tutoring (1)

