


Acids and Bases



ACT



AP



AP Art and Design


AP Chemistry



AP Physics 1


AP Physics 2


AP Physics C : E & M

AP Physics C: Mechanics


AP World History: Modern


Applications of Thermodynamics


AQA


Artificial intelligence (AI)

Atomic Structure and Properties


Banking and Finance

Basic Electronics

Biology


Business Ideas

Calculator


Capacitors


ChatGPT


Chemical Reactions


Chemistry


Circular Motion and Gravitation


Colleges Rankings


Computer Science


Conversion Tools


Cosmetic Procedures


Cryptocurrency

DC Circuits


Dynamics (Physics)


Edexcel

Electric Charge and Electric Force


Energy


English


Environmental Science
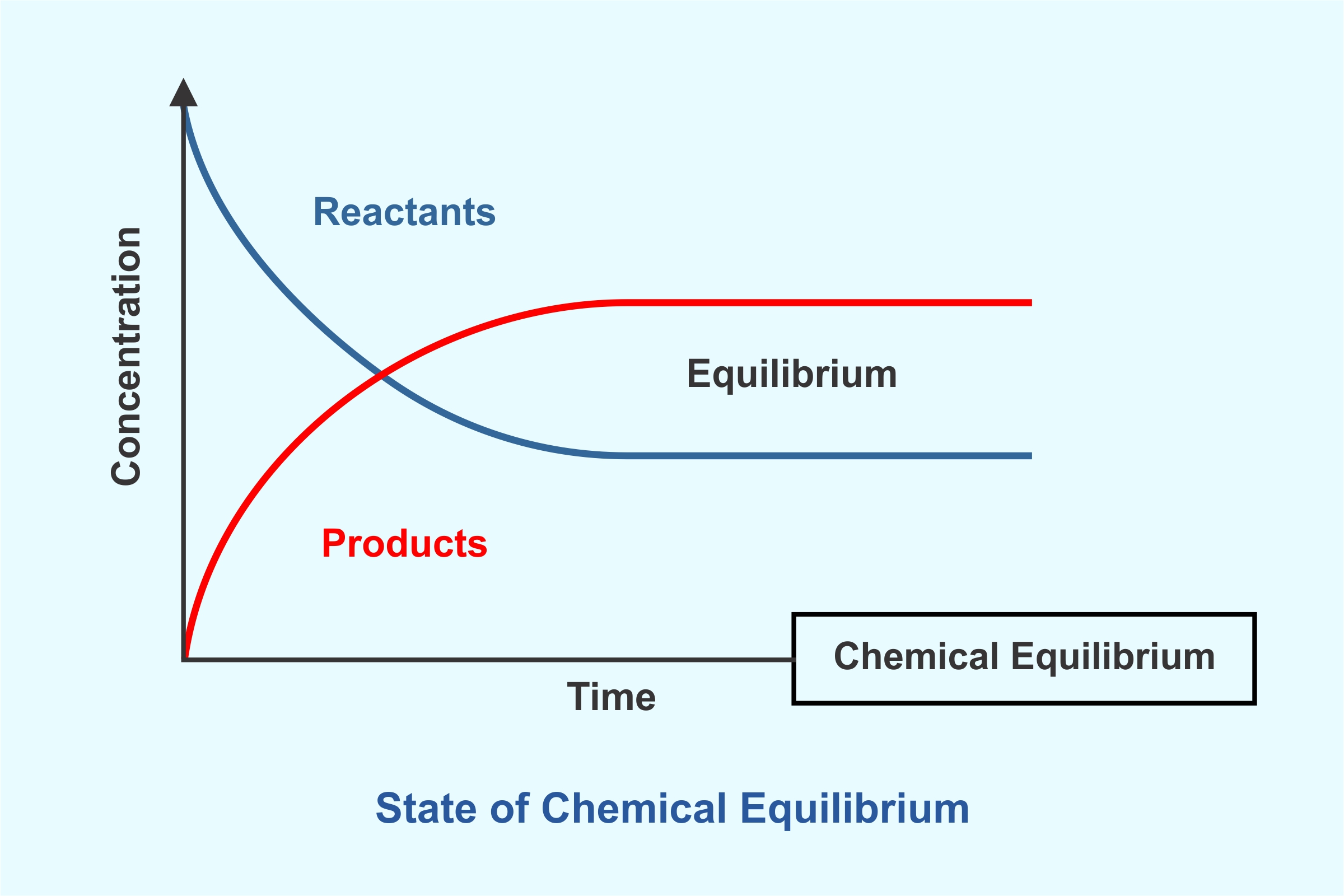

Equilibrium


Exam Skills


Exam Updates


Finance


Fitness & Wellness

Free Learning Resources


GCSE


General Guides


Health


History and Social Sciences


IB


IB BUSINESS MANAGEMENT SL


IGCSE


Image Converters


IMF


Intermolecular Forces and Properties

Kinematics


Kinematics (Physics)


Math


Mechanical Waves and Sound


Mental Health


Molecular and Ionic Compound Structure and Properties


Momentum


News


OCR


Past Papers

Periodic Table

Physics


SAT


Schools

Sciences


Short Notes


Simple Harmonic Motion


Study Guides


Syllabus


Thermochemistry


Tools

Torque and Rotational Motion


Tutoring


Unit 1: Electrostatics


Unit 1: Fluids

Unit 1: Kinematics


Unit 1: The Global Tapestry

Unit 2: Conductors, Capacitors, Dielectrics


Unit 2: Networks of Exchange


Unit 2: Newton’s Laws of Motion


Unit 2: Thermodynamics

Unit 3: Electric Circuits


Unit 3: Electric Force, Field, and Potential


Unit 3: Land-Based Empires


Unit 3: Work, Energy, and Power

Unit 4: Electric Circuits

Unit 4: Magnetic Fields


Unit 4: Systems of Particles and Linear Momentum


Unit 4: Transoceanic Interconnections

Unit 5: Electromagnetism

Unit 5: Magnetism and Electromagnetic Induction


Unit 5: Revolutions


Unit 5: Rotation


Unit 6: Consequences of Industrialization


Unit 6: Geometric and Physical Optics


Unit 6: Oscillations


Unit 7: Gravitation


Unit 7: Quantum, Atomic, and Nuclear Physics
AP Chemistry
AP Chemistry is a rigorous, college-level course that explores the principles of matter, energy, and chemical reactions. It covers topics like atomic structure, bonding, thermodynamics, equilibrium, kinetics, and electrochemistry. With a focus on inquiry-based learning, problem-solving, and lab experiments, AP Chemistry prepares students for the AP exam while building critical thinking and analytical skills. Ideal for those passionate about understanding the science behind chemical processes!


N
NUM8ERS
0
0
4.3 Representations of Reactions
November 14, 2024
Save


N
NUM8ERS
0
0
7.3 Reaction Quotient and Equilibrium Constant
November 18, 2024
Save


N
NUM8ERS
0
0
9.9 Cell Potential Under Nonstandard Conditions
November 18, 2024
Save

A
Admin
0
0
2.2 Intramolecular Force and Potential Energy
October 8, 2024
Save

N
NUM8ERS
0
0
7.14 Free Energy of Dissolution
November 18, 2024
Save

N
NUM8ERS
0
0
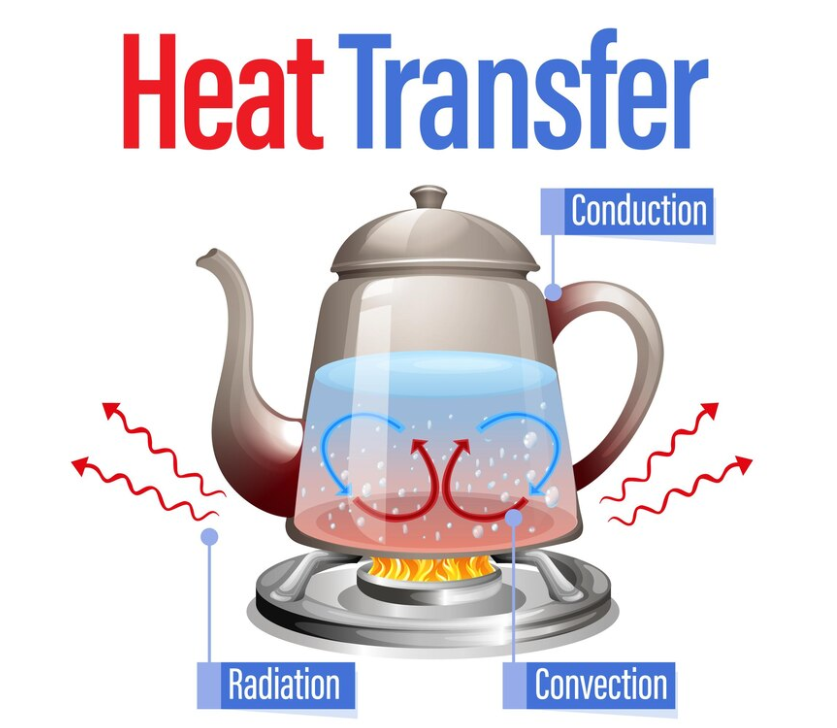
6.3 Heat Transfer and Thermal Equilibrium
November 18, 2024
Save


N
NUM8ERS
0
0
4.4 Physical and Chemical Changes
November 14, 2024
Save

N
NUM8ERS
0
0
7.4 Calculating the Equilibrium Constant
November 18, 2024
Save


N
NUM8ERS
0
0
9.10 Electrolysis and Faraday's Law
November 18, 2024
Save



