9.5 Free Energy and Equilibrium


Table of Contents
Toggle9.5 Free Energy and Equilibrium: The Relationship Explained
Kinetic vs. Thermodynamic Equilibrium: What’s the Difference?
In Unit 7, we explored equilibrium as the state where the forward and reverse reactions proceed at the same rate, leading to constant concentrations of reactants and products. This is known as the kinetic definition of equilibrium. But equilibrium also has a thermodynamic definition—it’s the state at which free energy (ΔG) is minimized. This understanding ties together spontaneity, ΔG, and equilibrium constants (K), providing a deeper insight into chemical reactions.
Kinetic Definition Recap
Equilibrium from a kinetic perspective means that, at equilibrium, the rate of the forward reaction equals the rate of the reverse reaction, resulting in unchanging concentrations of reactants and products. Importantly, this balance doesn’t mean reactions stop; both reactions continue at equal rates.
Thermodynamic Definition: The Role of Free Energy
From a thermodynamic perspective, equilibrium is reached at the minimum point of free energy (ΔG). When ΔG < 0, the reaction proceeds spontaneously, releasing free energy. Once the reaction reaches equilibrium, ΔG = 0, indicating no further net change in free energy without outside energy input. Beyond this point, the reaction becomes nonspontaneous (ΔG > 0) and would require external energy to proceed.
Graphical Representation of Free Energy and Equilibrium
Consider the graph below, where free energy (G) is plotted against the extent of the reaction (from 100% reactants to 100% products):
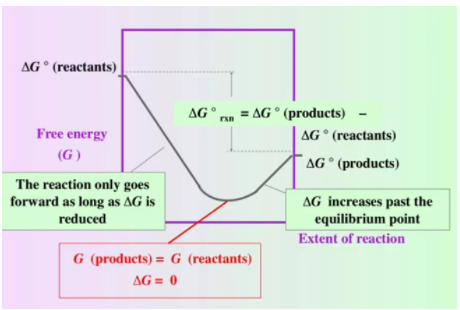

Image From Mr. Chemistry
Breaking Down the Graph
- Initial Conditions: On the left, we start with 100% reactants (ΔG = ΔG° of the reactants).
- Final Conditions: On the right, we end with 100% products (ΔG = ΔG° of the products).
- Spontaneous Reaction: For a reaction with ΔG° < 0, we move from reactants to products spontaneously until reaching equilibrium, where ΔG = 0.
- Nonspontaneous Reaction: Beyond equilibrium, ΔG > 0, meaning additional energy is needed to proceed further.
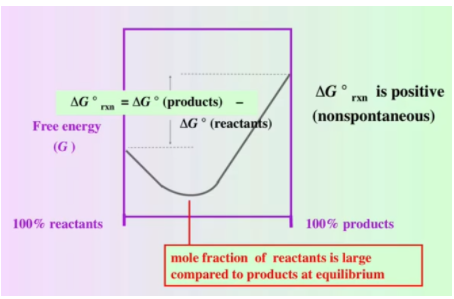

Image From Mr. Chemistry
Relating ΔG, ΔG°, and K
To quantify these relationships, we use the equation:
- Q is the reaction quotient, calculated using the concentrations or pressures of the reactants and products at any point.
- R is the gas constant (8.314 J/mol·K).
- T is the temperature in Kelvin.
At equilibrium, ΔG = 0 and Q = K (the equilibrium constant), simplifying the equation to:
Rearranging gives:
Or:
What This Means for Spontaneity and K
- If ΔG° < 0, the reaction is spontaneous and K > 1, favoring product formation.
- If ΔG° > 0, the reaction is nonspontaneous and K < 1, favoring reactants.
This relationship explains why reactions with highly negative ΔG° values proceed readily to products, while reactions with positive ΔG° values require external energy input to occur.
Key Takeaways
- Equilibrium can be defined both kinetically (equal rates of forward and reverse reactions) and thermodynamically (minimum free energy).
- ΔG° and K are linked mathematically, helping predict whether a reaction favors reactants or products at equilibrium.
- Understanding the relationship between free energy and equilibrium allows for deeper insights into reaction behavior and spontaneity.
Recent Comments


9.10 Electrolysis and Faraday's Law


9.9 Cell Potential Under Nonstandard Conditions


9.8 Cell Potential and Free Energy


9.7 Galvanic (Voltaic) and Electrolytic Cells


5.4 Conservation of Linear Momentum


5.3 Open and Closed Systems: Momentum

