

Table of Contents
Toggle9.1 Introduction to Entropy in Chemistry
Thermodynamics expands our understanding of energy and its movement within chemical reactions. In Unit 9, we take a closer look at entropy and Gibbs Free Energy, delving into the spontaneity of reactions. But first, what is entropy?
What is Entropy?
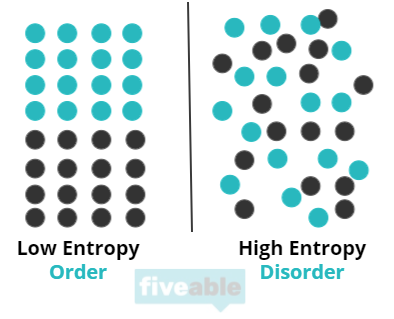
Entropy measures the “disorder” or “chaos” within a system. It answers, “How ordered is this system?” A higher entropy value indicates a more disordered state, while a lower value suggests a more ordered state.
Everyday Entropy: A Practical Example
Picture your bedroom on a chaotic day. You’ve thrown clothes everywhere, left books open, and tossed your sheets around. It takes no energy for the room to reach this state of disorder—entropy increases naturally. However, when it’s time to clean and organize your space, you exert energy. This effort to reduce disorder shows a decrease in entropy. This concept aligns with the Second Law of Thermodynamics: systems tend toward greater entropy.

Entropy and States of Matter
The connection between entropy and the states of matter is straightforward:
- Solids have the least entropy due to their tightly packed structure.
- Liquids have more entropy as their molecules can move more freely.
- Gases have the highest entropy; molecules move rapidly and with greater randomness.
Consider this progression:
Solid (s) ⇌ Liquid (l) ⇌ Gas (g)
Moving left to right (solid to gas) increases entropy, while the reverse decreases entropy.
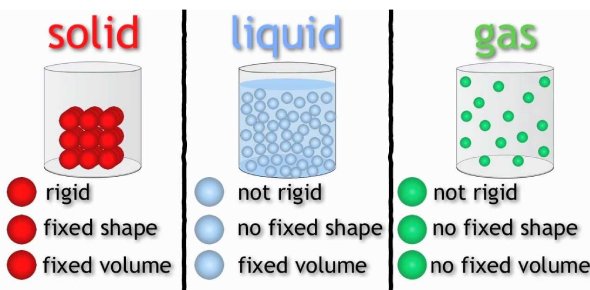

Image From ProProfs
The Three Laws of Thermodynamics
Law #1: Conservation of Energy
Also known as the First Law of Thermodynamics, this states that energy cannot be created or destroyed, only transformed. For example, when chemical energy becomes heat, the total energy remains constant.
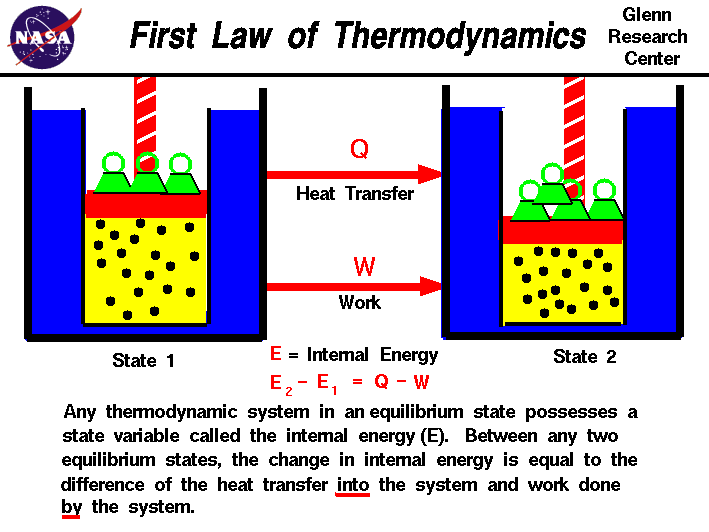

Image From NASA
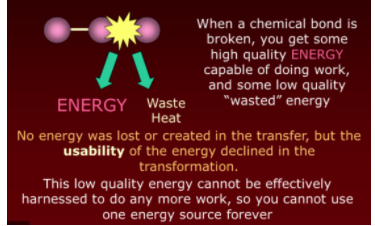
Law #2: Entropy and Energy Quality in Isolated Systems
This law has two main parts:
- Energy Quality: As energy transforms, some is inevitably lost as heat. Think of a power plant generating electricity; friction causes some energy loss as heat.
- Entropy Changes: In any isolated system, entropy tends to increase or remain constant. Spontaneous processes always result in an overall ΔS ≥ 0.
Image From SlidePlayer
Law #3: Absolute Zero
The Third Law states that at absolute zero (0 K), entropy is zero. This is because, at this temperature, all molecular motion halts, leaving no disorder.
Entropy and Spontaneity in Reactions
The concept of spontaneity relates to whether a reaction will proceed without outside intervention:
- Spontaneous processes: No extra energy needed, like a ball rolling downhill.
- Non-spontaneous processes: Require energy input, like pushing a ball uphill.
Calculating Entropy Changes
Chemists use ΔS values to calculate changes in entropy for reactions. A positive ΔS means increasing disorder, while a negative ΔS signifies a shift to more order. Understanding this helps predict reaction behavior.
Recent Comments


9.10 Electrolysis and Faraday's Law


9.9 Cell Potential Under Nonstandard Conditions


9.8 Cell Potential and Free Energy


9.7 Galvanic (Voltaic) and Electrolytic Cells


5.3 Open and Closed Systems: Momentum

