

Table of Contents
ToggleUnderstanding pH and pKa: Key Concepts for Acid-Base Chemistry
In AP Chemistry, mastering the concepts of pH and pKa is crucial for navigating acid-base equilibrium, titrations, and buffers. This guide will break down the relationship between these measures, how they relate to acid strength, and their importance in buffer systems and titrations.
What Does ‘p’ Notation Mean?
In acid-base chemistry, you’ll frequently encounter ‘p’ notation for various measures, including pH, pKa, pOH, and pKb. This notation simply represents the negative logarithm of a value:
- pH = -log[H+] (measuring the concentration of hydrogen ions, or acidity)
- pOH = -log[OH-] (measuring the concentration of hydroxide ions, or basicity)
- pKa = -log(Ka) (measuring the acid dissociation constant)
The ‘p’ notation transforms values into a logarithmic scale, making it easier to work with small numbers that vary across many orders of magnitude.
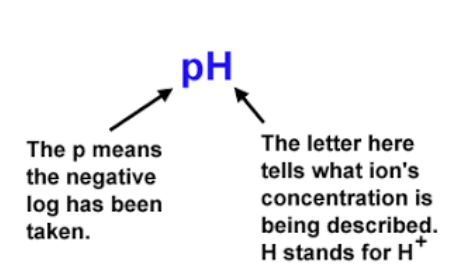

Image from UIUC
pKa and Acid Strength: Decoding the Relationship
The pKa value of an acid reflects its strength in water. Lower pKa values correspond to stronger acids, which dissociate more completely to release H+ ions. Conversely, a higher pKa indicates a weaker acid.
For example:
- Acid A has a pKa of 2.
- Acid B has a pKa of 3.
Acid A is 10 times stronger than Acid B, as pKa is on a logarithmic scale. However, it’s important to note that a high pKa does not necessarily imply a basic nature; it only shows a relatively weaker tendency to donate protons.
pKa + pKb = 14 is another key relationship to remember, linking pKa of an acid and pKb of its conjugate base in water at 25°C.
Connecting pH and pKa in Buffers
Buffers are solutions that resist pH changes, typically comprising a weak acid and its conjugate base. The relationship between pH and pKa can be captured using the Henderson-Hasselbalch Equation:
pH=pKa+log([HA][A−])
The optimal buffering capacity occurs when [A-] = [HA], making pH = pKa. This point is especially important in titration curves, as it represents the half-equivalence point, where the solution acts as the strongest buffer.
Choosing the Right Acid-Base Indicator
Acid-base indicators are compounds that change color based on pH. They are commonly used in titrations to signal the equivalence point, where the amounts of acid and base are stoichiometrically equal. Common indicators include bromothymol blue, phenolphthalein, and methyl red.
Selecting an Indicator:
- The indicator’s effective range should align with the expected pH range of the reaction.
- The effective range is typically pKa ± 1 of the indicator.
- For example, if a titration’s equivalence point pH is 7, you would choose an indicator with a range that encompasses pH 7.
Real-Life Example:
In an acid-base titration with a strong acid and strong base, the equivalence point is usually at pH 7. An appropriate indicator would be one with a transition range around pH 7, such as methyl red.
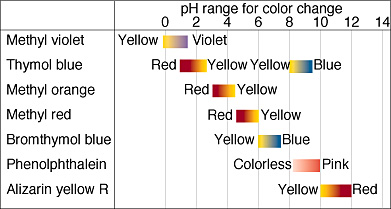

Image From Prenhall
Practical Application: Example Problem
Example:
Question: Find the best indicator for a titration with an equivalence point of pH 7. Solution: Since the pH at the equivalence point is 7, you should choose an indicator that changes color around this pH value. Methyl red fits this criterion and would be a suitable choice.
Key Takeaways
- pKa indicates the strength of an acid, with lower values representing stronger acids.
- pH and pKa are crucial for buffer solutions, particularly at the half-equivalence point where pH = pKa.
- Acid-base indicators help visualize titration progress, with their effectiveness determined by their pKa and color-change range.


8.9 Henderson-Hasselbalch Equation


3.8 Applications of Circular Motion and Gravitation


3.7 Free-Body Diagrams for Objects in Uniform Circular Motion


3.6 Centripetal Acceleration and Centripetal Force

