

Table of Contents
ToggleUnderstanding pH and pOH of Strong Acids and Bases: Essential Guide for Chemistry
pH and pOH are fundamental concepts in acid-base chemistry that help us understand the acidity and basicity of solutions. They measure the concentrations of hydrogen ions (H⁺) and hydroxide ions (OH⁻), respectively. In this guide, we will explore what pH and pOH mean, how they are related, and how to find the pH and pOH of strong acids and bases.
What is pH?
pH is a scale that measures the concentration of hydrogen ions (H⁺) in a solution. It indicates how acidic or basic a solution is. The pH scale ranges from 0 to 14:
- pH < 7: Acidic solution
- pH = 7: Neutral solution (e.g., pure water)
- pH > 7: Basic solution
The pH of a solution is calculated using the formula: pH = −log[H⁺]
Example Calculation:
For a solution with an H⁺ concentration of 0.01 M, the pH is: pH = −log(0.01) = 2.
This means the solution is acidic.
What is pOH?
pOH is a measure of the concentration of hydroxide ions (OH⁻) in a solution. It complements pH and is calculated as: pOH = −log[OH⁻]
A low pOH indicates a highly basic (alkaline) solution, while a high pOH indicates a less basic solution.
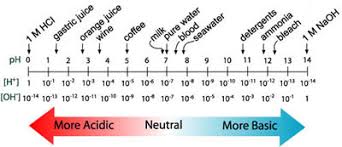

Relationship Between pH and pOH
pH and pOH are related through the autoionization of water: H₂O ⇌ H⁺ + OH⁻
The equilibrium constant for this reaction is known as Kw: Kw = [H⁺][OH⁻] = 1.0 × 10⁻¹⁴ at 25°C
Taking the negative logarithm of both sides gives: pH + pOH = 14
This relationship allows you to find pH if you know pOH, and vice versa.
Calculating pH and pOH of Strong Acids and Bases
Strong acids and bases completely dissociate in water. This means their concentrations can be directly used to find the pH or pOH.
Example: Strong Acid (HCl)
Consider a 1 M solution of HCl. The dissociation reaction is: HCl → H⁺ + Cl⁻
Since HCl completely dissociates, the concentration of H⁺ is 1 M. The pH is calculated as: pH = −log(1) = 0
Example: Strong Base (NaOH)
Consider a 1 M solution of NaOH. The dissociation reaction is: NaOH → Na⁺ + OH⁻
Since NaOH completely dissociates, the concentration of OH⁻ is 1 M. The pOH is: pOH = −log(1) = 0
To find the pH: pH = 14 − pOH = 14 − 0 = 14
List of Strong Acids
There are seven strong acids you need to memorize for AP Chemistry. These acids completely ionize in water, making them key examples of strong acids:
- Hydrochloric acid (HCl)
- Hydrobromic acid (HBr)
- Hydroiodic acid (HI)
- Nitric acid (HNO₃)
- Sulfuric acid (H₂SO₄)
- Chloric acid (HClO₃)
- Perchloric acid (HClO₄)
For these acids, the concentration of H₃O⁺ (or H⁺) in solution equals the initial concentration of the acid, making pH calculations straightforward.
Key Takeaways
- pH measures H⁺ concentration; pOH measures OH⁻ concentration.
- pH + pOH = 14 at 25°C.
- Strong acids and bases fully dissociate, simplifying pH and pOH calculations.
- Memorize the seven strong acids for quick pH calculations.


8.9 Henderson-Hasselbalch Equation


3.8 Applications of Circular Motion and Gravitation


3.7 Free-Body Diagrams for Objects in Uniform Circular Motion


3.6 Centripetal Acceleration and Centripetal Force

