

Table of Contents
ToggleUnit 7 Overview: Equilibrium
Introduction to Equilibrium in AP Chemistry
Equilibrium is a fundamental concept in chemistry where the rate of the forward reaction equals the rate of the reverse reaction. Contrary to what you may think, reactions at equilibrium do not stop; rather, they reach a state where the concentrations of reactants and products remain constant over time.
In this unit, you’ll learn about the qualitative and quantitative aspects of equilibrium, including key principles like Le Châtelier’s Principle, which explains how a system at equilibrium responds to external changes. You’ll also delve into equilibrium constants (K), reaction quotients (Q), solubility equilibria, and their practical applications.
Key Topics in Unit 7
7.1 Introduction to Equilibrium
Equilibrium occurs when the forward and reverse reaction rates are equal. This dynamic state is characterized by constant concentrations of reactants and products. The study of equilibrium focuses on understanding how and why reactions reach this state.7.2 Direction of Reversible Reactions
Reversible reactions can move in both the forward (reactants → products) and reverse (products → reactants) directions. We use double arrows (⇌) to denote this in chemical equations. At equilibrium, the concentrations of reactants and products become stable, even though both reactions continue to occur at the same rate.
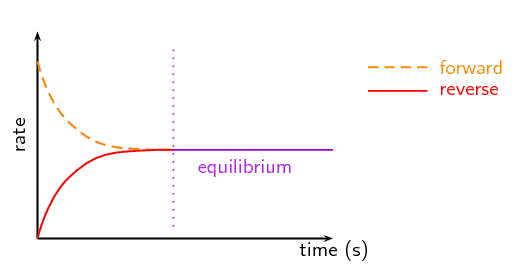
Image From Siyavula
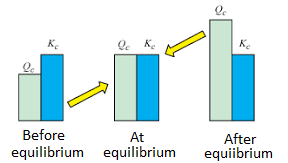
7.3 Reaction Quotient (Q) and Equilibrium Constant (K)
- Q measures the ratio of products to reactants at any point during a reaction.
- K describes this ratio at equilibrium.
Comparing Q to K indicates whether a reaction will shift forward or backward to reach equilibrium.
Image Courtesy of Byjus
7.4 Calculating the Equilibrium Constant
Use the formula:Only gases and aqueous species are included in the equilibrium expression. Solids and pure liquids are excluded.

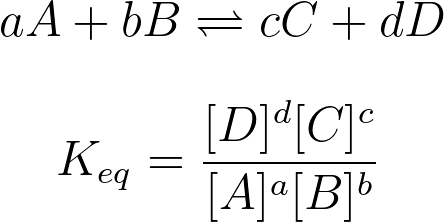
Image Courtesy of Study.com
7.5 Magnitude of the Equilibrium Constant
- K > 1: Product-favored reaction (more products at equilibrium).
- K < 1: Reactant-favored reaction (more reactants at equilibrium).
- K = 1: Neither direction is favored.
7.6 Properties of the Equilibrium Constant
- Reversing a reaction inverts K.
- Multiplying a reaction by a coefficient raises K to that power.
- Adding reactions results in multiplying their K values.
7.7 Calculating Equilibrium Concentrations
Use ICE tables (Initial, Change, Equilibrium) to determine the concentrations of reactants and products at equilibrium using K values.
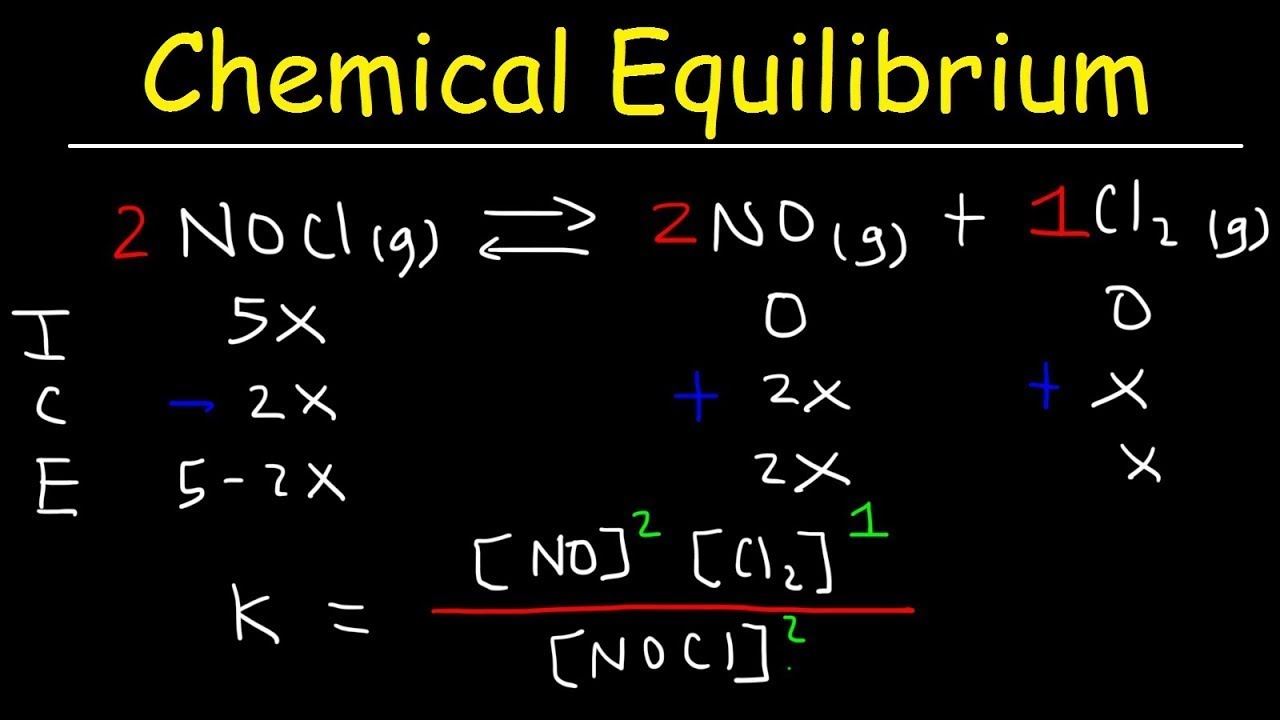
Image Courtesy of the Organic Chemistry Tutor
7.8 Representations of Equilibrium
Equilibrium can be represented mathematically, graphically, or using particle models. Understanding these representations is crucial for interpreting and predicting how systems respond to changes.7.9 Le Châtelier’s Principle
When a system at equilibrium is disturbed by changes in concentration, temperature, or pressure, it will shift to counteract the disturbance and restore equilibrium.

Image Courtesy of SlideShare
7.10 Reaction Quotient (Q) and Le Châtelier’s Principle
Compare Q to K to predict the direction of the reaction shift when a system is stressed. Temperature changes, however, alter K itself, unlike changes in concentration or pressure.7.11 Introduction to Solubility Equilibria
Solubility equilibrium involves the dissolution of substances in water. Even insoluble substances establish a tiny equilibrium constant (Ksp).7.12 Common Ion Effect
Adding a common ion reduces the solubility of a compound by shifting the equilibrium position, which can be analyzed using ICE tables.
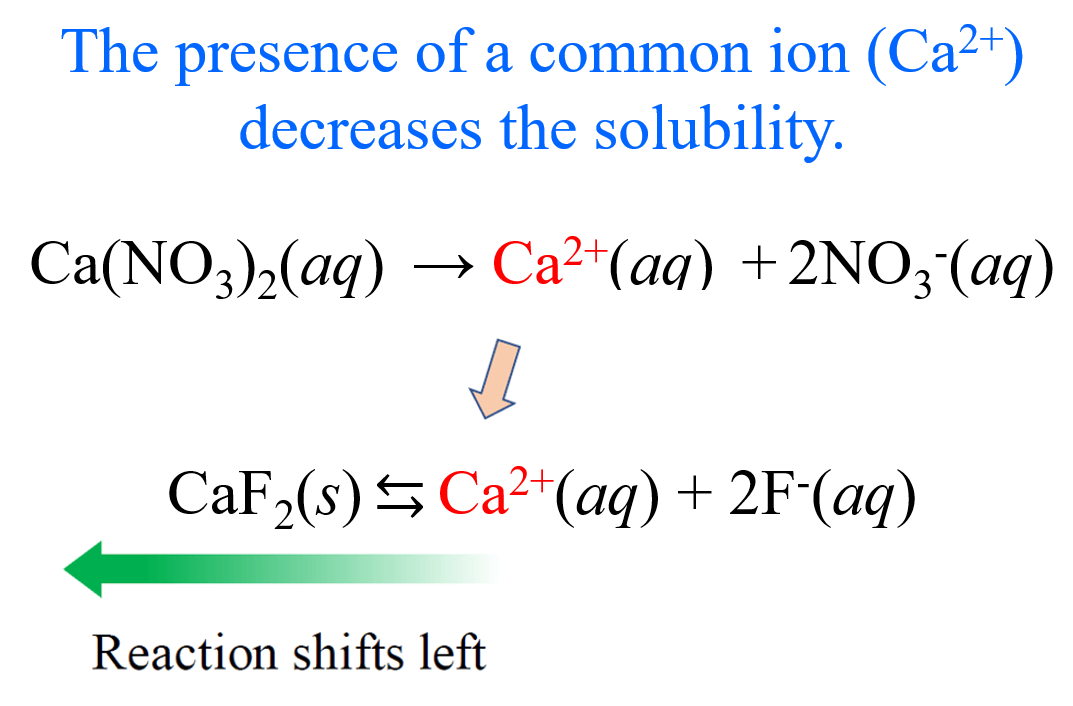
Image Courtesy of Chemistry Steps
7.13 pH and Solubility
Changes in pH can influence solubility equilibria, especially for salts that produce or react with H+ or OH- ions.7.14 Free Energy of Dissolution
Explore how enthalpy (ΔH) and entropy (ΔS) changes during dissolution relate to the free energy change (ΔG), giving insights into the solubility process.
Important Vocabulary
- Equilibrium: State where the forward and reverse reaction rates are equal, and reactant and product concentrations remain constant.
- Reversible Reaction: A chemical reaction that proceeds in both directions.
- Equilibrium Constant (K): Ratio of product to reactant concentrations at equilibrium.
- Reaction Quotient (Q): Ratio of product to reactant concentrations at any point.
- Le Châtelier’s Principle: States that a system at equilibrium will adjust to counteract any imposed change.
- Common Ion Effect: Describes how the solubility of a compound is affected by the addition of a common ion.
Practical Applications and Real-World Examples
- Why is food refrigerated?
Lowering temperature slows the rate of reactions, helping food last longer. - How is caffeine removed from coffee?
Le Châtelier’s Principle and equilibrium concepts are applied to remove caffeine via solvents.


7.14 Free Energy of Dissolution


7.11 Introduction to Solubility Equilibria


3.8 Applications of Circular Motion and Gravitation


3.7 Free-Body Diagrams for Objects in Uniform Circular Motion


3.6 Centripetal Acceleration and Centripetal Force

