

Table of Contents
Toggle7.5 Magnitude of the Equilibrium Constant
What is the Equilibrium Constant?
In previous sections, we explored what equilibrium is and how to represent it mathematically using equilibrium constants like Kc (based on concentrations) and Kp (based on partial pressures). But what exactly does the magnitude of K tell us about a reaction? This section will provide a deeper dive into interpreting the value of the equilibrium constant and what it signifies for chemical reactions.
What Does the Equilibrium Constant (K) Tell Us?
The equilibrium constant represents the ratio of the concentration of products to reactants at equilibrium, with each raised to the power of their stoichiometric coefficients. This means K gives us a snapshot of how “far forward” a reaction proceeds before reaching equilibrium.
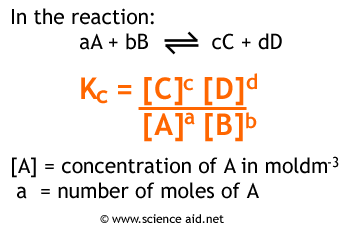
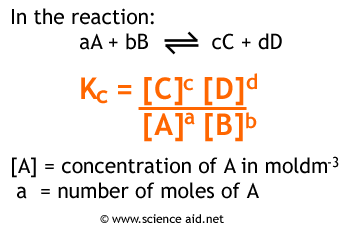
Image Courtesy of ScienceAid
Key Points to Remember:
- If K > 1: The reaction is product-favored. More products are formed at equilibrium compared to the amount of reactants remaining. The larger the value of K, the more the reaction proceeds towards the products.
- If K = 1: The reaction is in a state of balance between reactants and products, meaning neither is favored over the other.
- If K < 1: The reaction is reactant-favored. Only a small amount of product forms, with most reactants remaining at equilibrium.
Breaking Down the Formula
Consider the general form of the equilibrium constant expression for a reaction:
aA + bB ⇌ cC + dD
K = [C]^c[D]^d / [A]^a[B]^b
- The numerator represents the concentration (or partial pressure) of products.
- The denominator represents the concentration (or partial pressure) of reactants.
This ratio directly shows whether products or reactants are more dominant at equilibrium. Here’s what this means practically:
- When K is large: The numerator (products) is significantly larger than the denominator (reactants). This implies that most of the reactants have been converted into products.
- When K is small: The numerator (products) is smaller, indicating that very little product is formed.
Comparing Reactions Using K
Example 1:
Consider two acid dissociation reactions:
- CH₃COOH ⇌ CH₃COO⁻ + H⁺ with K = 1.8 × 10⁻⁵
- HCl ⇌ Cl⁻ + H⁺ with K = 1.3 × 10⁶
Which releases more H⁺ into the solution?
- Since the K value for the dissociation of HCl is much greater than 1, it is heavily product-favored, meaning it dissociates almost completely. By contrast, CH₃COOH has a K value far less than 1, indicating most of it remains undissociated. Thus, HCl releases far more H⁺ ions.
Chloride and H3O+, the products of the dissociation of HCl. Image from Wikipedia
Practice Problem: Identifying Reaction Favorability
Identify each of the following reactions as either product-favored or reactant-favored:
- CH₃COOH ⇌ CH₃COO⁻ + H⁺ with K = 1.8 × 10⁻⁵
- 2O₃ ⇌ 3O₂ with K = 2.5 × 10¹²
Analysis:
Reaction 1 (Acetic Acid Dissociation)
Since K < 1, this reaction is reactant-favored. Only a small fraction of acetic acid dissociates, resulting in a solution that is primarily CH₃COOH.Reaction 2 (Ozone Decomposition)
With K > 1, this reaction is product-favored. The reaction proceeds nearly to completion, meaning most O₃ decomposes to form O₂.
Real-Life Example: Ozone Decomposition and Environmental Impact
The reaction 2O₃ ⇌ 3O₂ occurs in the Earth’s atmosphere and is catalyzed by chlorine (from chlorofluorocarbons or CFCs). When chlorine catalyzes this reaction, it accelerates the breakdown of O₃ (ozone), contributing to the depletion of the ozone layer, which is crucial for shielding life from harmful ultraviolet radiation.
Key Takeaways
- The magnitude of the equilibrium constant tells us about the relative amounts of products and reactants at equilibrium.
- K > 1 means a product-favored reaction; K < 1 means a reactant-favored reaction.
- The greater the magnitude of K, the further the reaction proceeds in the forward direction.
- Understanding the magnitude of K allows for predictions about how much product forms in a reaction.


7.14 Free Energy of Dissolution


7.11 Introduction to Solubility Equilibria


3.8 Applications of Circular Motion and Gravitation


3.7 Free-Body Diagrams for Objects in Uniform Circular Motion


3.6 Centripetal Acceleration and Centripetal Force

