

Table of Contents
Toggle7.1 Introduction to Equilibrium
Understanding Reversible Reactions and Equilibrium
Welcome to the introduction to equilibrium! Equilibrium is a critical concept in chemistry, not just for the AP exam but for understanding reactions at a fundamental level. This unit builds upon knowledge from previous units, particularly how reactions can proceed both forward (from reactants to products) and backward (from products back to reactants). Here, we explore how these reversible reactions establish equilibrium and how we can measure and influence their extent.

Image From GIPHY
What Are Reversible Reactions?
Reversible reactions can proceed in both the forward direction (forming products from reactants) and the reverse direction (forming reactants from products). We represent these reactions with a double arrow (⇌), indicating that both reactions occur simultaneously:
- Examples of reversible reactions:
- Evaporation and condensation of water: H₂O(l) ⇌ H₂O(g)
- Dissolution and precipitation of a salt: NH₄Cl(s) ⇌ NH₄⁺(aq) + Cl⁻(aq)
- Acid-base reactions: H₂CO₃ + HCO₃⁻ ⇌ H₂O + CO₂
- Redox reactions: Zn + Cu²⁺ ⇌ Zn²⁺ + Cu
These reactions can reach a state where the forward and reverse processes happen at the same rate, leading to equilibrium.
Understanding Equilibrium
Equilibrium is the state in which the rate of the forward reaction equals the rate of the reverse reaction. Here’s what that means:
Dynamic State: At equilibrium, reactions do not stop; they continue to occur in both directions at equal rates, maintaining constant concentrations of reactants and products.
Graphical Representation:
The graph below illustrates this concept for the reaction H₂ + I₂ ⇌ 2HI:
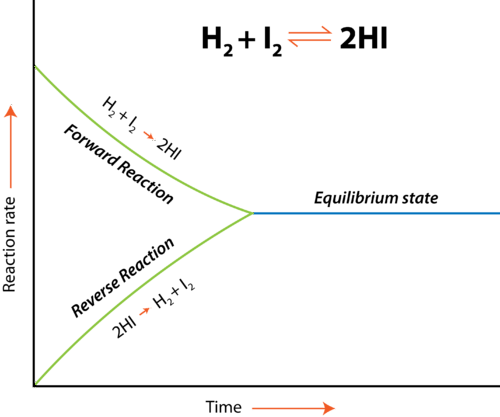
Image From LibreTexts/Chapters/Chapter_8%3A_Properties_of_Solutions/8.2%3A_Chemical_Equilibrium)
As shown, the forward and reverse reaction rates converge at equilibrium. While the rates are equal, the concentrations of reactants and products are constant (though not necessarily equal).
The Importance of a Closed System
Equilibrium can only be achieved in a closed system—a system that does not exchange matter or energy with its surroundings. In an open system, the exchange of matter or energy can alter reactant and product concentrations, preventing the establishment of equilibrium.
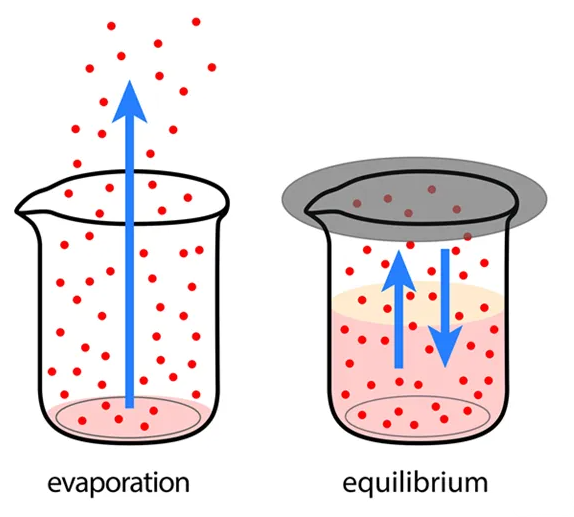

Image Courtesy of Shalom Education
Measuring Equilibrium with the Equilibrium Constant (K)
The equilibrium constant (K) quantifies how far a reaction proceeds toward products before reaching equilibrium. For a general reaction A ⇌ B:
- Forward reaction rate: k₁[A]
- Reverse reaction rate: k₂[B]
At equilibrium, k₁[A] = k₂[B], and we define K as:
Key Points:
- K is unitless.
- K is temperature-dependent, but initial reactant/product concentrations do not affect its value.
Partial Pressures and Kp
For gas-phase reactions, we often use Kp, which uses partial pressures instead of concentrations. Kp and Kc are related through:
where Δn is the difference in moles of gaseous products and reactants.
Common Misconception: Equilibrium Means No Reaction
A major misconception is that reactions stop at equilibrium. In reality, equilibrium is a dynamic state where forward and reverse reactions continue at equal rates. Concentrations remain constant, but the system remains active.
Conclusion
Understanding equilibrium is key to mastering chemical reactions and their behaviors. This foundational concept allows you to predict and control reaction outcomes, both qualitatively and quantitatively.
Key Takeaways
- Reversible Reactions: Many chemical reactions are reversible, establishing a dynamic equilibrium.
- Equilibrium State: Occurs when forward and reverse reaction rates are equal.
- Equilibrium Constant (K): Quantifies how far a reaction proceeds toward products at equilibrium.
- Closed System Requirement: Equilibrium is only achieved in closed systems.
- Dynamic Process: Reactions continue at equilibrium; they do not stop.
Recent Posts
- 6.2 Energy of a Simple Harmonic Oscillator
- 6.1 Period of Simple Harmonic Oscillators
- Unit 6 Overview: Simple Harmonic Motion
- 5.4 Conservation of Linear Momentum
- 5.3 Open and Closed Systems: Momentum
- 5.2 Representations of Changes in Momentum
- Columbia University
- 5.1 Momentum and Impulse
- Unit 5 Overview: Momentum
- 4.3 Conservation of Energy, the Work-Energy Principle, and Power
- 4.2 Work and Mechanical Energy
- 4.1 Open and Closed Systems: Energy
- Unit 4 – Energy
- Premier Tutoring Excellence in Dubai, UAE 📚✨
- How to Open a Non-Resident Bank Account in UAE – Complete Guide
Choose Topic
- ACT (15)
- AP (20)
- AP Art and Design (5)
- AP Physics 1 (1)
- AQA (5)
- Banking and Finance (1)
- Biology (13)
- Business Ideas (68)
- Calculator (67)
- Chemistry (3)
- Colleges Rankings (8)
- Computer Science (4)
- Conversion Tools (135)
- Cosmetic Procedures (50)
- Cryptocurrency (49)
- Edexcel (4)
- English (1)
- Environmental Science (2)
- Exam Updates (1)
- Finance (17)
- Fitness & Wellness (164)
- Free Learning Resources (184)
- GCSE (1)
- Health (107)
- History and Social Sciences (8)
- IB (1)
- IGCSE (2)
- Image Converters (3)
- IMF (10)
- Math (39)
- Mental Health (58)
- OCR (3)
- Past Papers (463)
- Physics (5)
- SAT (36)
- Sciences (1)
- Short Notes (5)
- Study Guides (26)
- Syllabus (19)
- Tutoring (1)
Recent Comments


7.14 Free Energy of Dissolution


7.11 Introduction to Solubility Equilibria


6.2 Energy of a Simple Harmonic Oscillator


6.1 Period of Simple Harmonic Oscillators


Unit 6 Overview: Simple Harmonic Motion

