

Table of Contents
Toggle6.7 Bond Enthalpies: Understanding Bond Energy in Chemical Reactions
Bond Energetics: The Basics
Every chemical bond contains energy that plays a critical role in determining whether a reaction is endothermic (absorbs heat) or exothermic (releases heat). In simple terms, breaking chemical bonds requires energy, while forming new bonds releases energy. This concept is central to understanding reaction energetics.
Quick Mnemonic: BARF
- Breaking Absorbing – Releasing Forming (BARF):
- Breaking bonds absorbs energy.
- Forming bonds releases energy.
Breaking Bonds: Energy Required
To break a bond, energy must be input into the system. Think of it like trying to snap a rubber band—energy is needed to stretch it before it breaks. Similarly, to separate bonded atoms, energy is absorbed by the system. For example, breaking a single H-H bond in a molecule requires energy input.
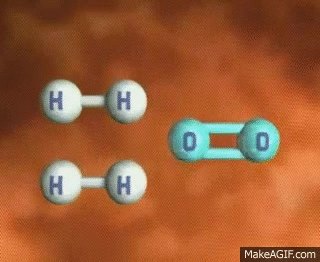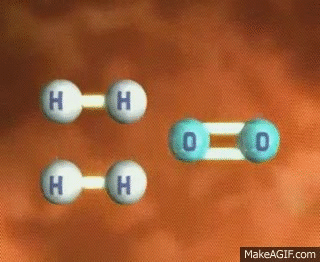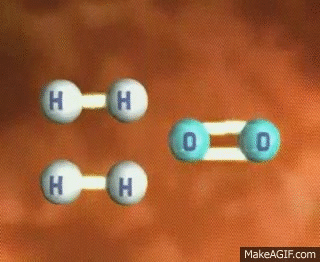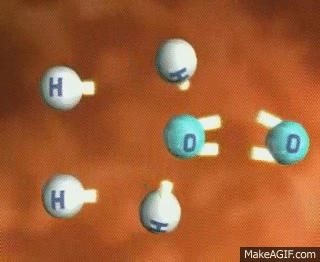

GIF Courtesy of MakeAGIF
Making Bonds: Energy Released
When bonds form, energy is released because the resulting molecules are in a lower potential energy state. This excess energy is released from the system. In simpler terms, atoms release energy when they form stable bonds.
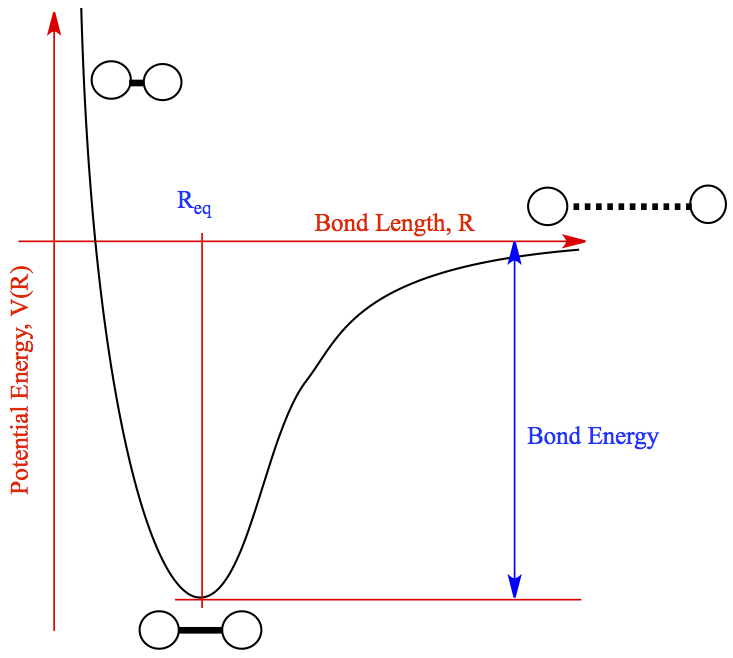

Bond Dissociation Energy (BDE)
Bond Dissociation Energy (BDE) refers to the energy needed to break one mole of a specific bond in the gas phase. This value varies across different bonds. Here are some important trends to remember:
- More Bonds, Higher BDE:
- Triple bonds > Double bonds > Single bonds.
- Example: Breaking a C≡C triple bond requires more energy than breaking a C=C double bond, which in turn requires more energy than breaking a C-C single bond.
- Longer Bonds, Weaker Bonds:
- Shorter bonds are stronger and require more energy to break, while longer bonds are weaker and have lower BDEs.
- Example: C-H bonds are shorter and stronger compared to longer, weaker C-C bonds.
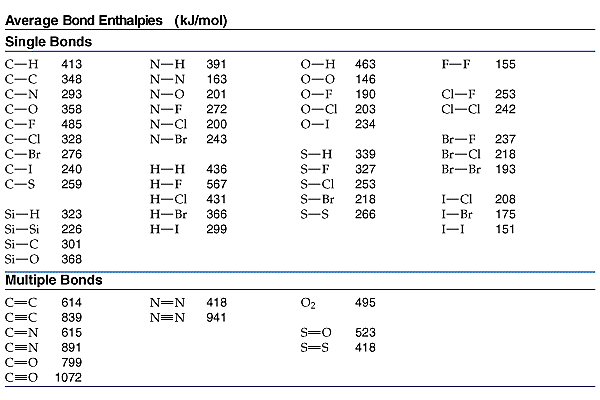

Using BDEs to Calculate Enthalpy of Reaction (ΔH)
To determine the enthalpy change (ΔH) of a reaction using bond dissociation energies, we use the following formula:
Important Reminder:
- ΔH = Bonds Broken – Bonds Formed (Reactants – Products).
- This formula works because a chemical reaction involves breaking reactant bonds and forming product bonds.
Example Calculation 1
Reaction:
BDE Data:
- H-H bond: 432 kJ/mol
- O=O bond: 498 kJ/mol
- O-H bond: 463 kJ/mol
Step-by-Step Calculation:
- Calculate energy needed to break reactant bonds:
- Calculate energy released in forming product bonds:
- Apply the formula:
- Result: The reaction is exothermic as it releases 490 kJ/mol of energy.
Example Calculation 2
Reaction:
BDE Data:
- C-H: 413 kJ/mol
- O-H: 463 kJ/mol
- C-O: 358 kJ/mol
- C=O: 799 kJ/mol
- O=O: 498 kJ/mol
Calculation:
- Breaking Bonds (Reactants):
- Forming Bonds (Products):
- Enthalpy Change:
- Result: This reaction is exothermic, releasing 802 kJ/mol of energy.
Quick Summary and Key Takeaways
- Bonds Require Energy to Break and Release Energy When Formed.
- Shorter Bonds are stronger and have higher BDEs compared to longer, weaker bonds.
- ΔH Calculation using BDE: Always remember broken – formed (Reactants – Products).
- Use accurate drawings and bond structures to ensure the correct BDE values are applied in calculations.


6.6 Introduction to Enthalpy of Reaction


3.8 Applications of Circular Motion and Gravitation


3.7 Free-Body Diagrams for Objects in Uniform Circular Motion


3.6 Centripetal Acceleration and Centripetal Force

