

Table of Contents
Toggle6.6 Introduction to Enthalpy of Reaction
What is Enthalpy?
Enthalpy (H) is a key concept in thermochemistry that describes the total internal energy of a system, including the energy needed to change its temperature and pressure. In simple terms, enthalpy refers to the heat content of a system. When discussing chemical reactions, we focus on the change in enthalpy (ΔH), which is the difference between the enthalpy of the products and the reactants.
- ΔH > 0 (Positive): The reaction absorbs heat (endothermic).
- ΔH < 0 (Negative): The reaction releases heat (exothermic).
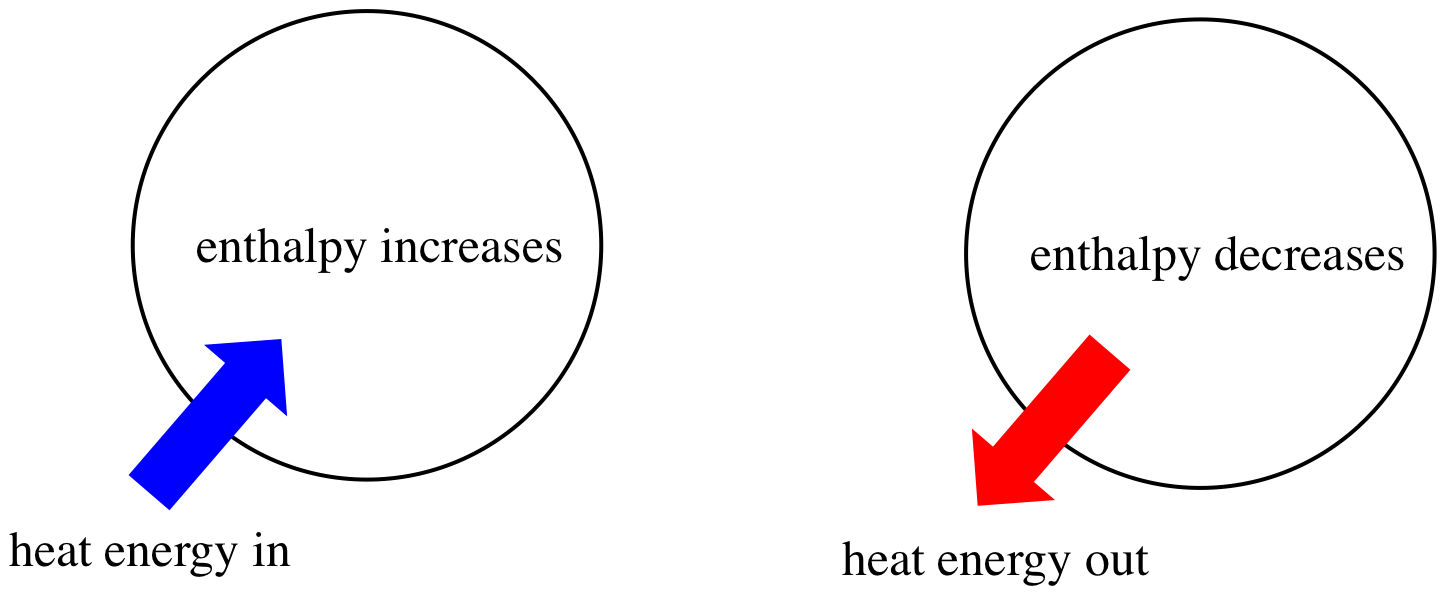

Image Courtesy of Chemistry
Enthalpy and Reaction Energy
The enthalpy of reaction (ΔH) describes the energy change during a chemical reaction:
- Exothermic Reactions: Heat is released, resulting in a negative ΔH. Example: The combustion of propane releases heat into the surroundings, often seen as flames.
- Endothermic Reactions: Heat is absorbed, resulting in a positive ΔH. Example: The dissolution of anhydrous copper sulfate in water causes the solution to become colder as it absorbs heat.
Key Examples:
Combustion of Propane (Exothermic):
- ΔH < 0; heat is released, observable as an increase in temperature or a flame.
Dissolution of Anhydrous CuSO₄ (Endothermic):
- ΔH > 0; heat is absorbed, observable as a temperature drop.
Positive vs. Negative ΔH
The sign of ΔH provides critical insight into the reaction’s heat flow direction:
- Positive ΔH (Endothermic): Heat flows into the system from the surroundings.
- Negative ΔH (Exothermic): Heat flows out of the system into the surroundings.
Tip: While the temperature change in the surroundings can provide clues about a reaction’s enthalpy change, the total energy exchange depends on the system’s internal energy changes, not just temperature shifts.
Example Reactions:
- Exothermic Reaction: Combustion of methane (CH₄) to form CO₂ and H₂O.
- ΔH < 0, indicating heat release.
- Endothermic Reaction: Melting of ice at 0°C.
- ΔH > 0, indicating heat absorption.
Thermodynamic Feasibility of Reactions
While a negative ΔH (exothermic reaction) often suggests a thermodynamically favorable reaction, it’s not the sole determining factor. The Gibbs free energy change (ΔG) and the equilibrium constant (Kc) also play crucial roles.
ΔG = ΔH – TΔS, where:
- ΔS is the change in entropy.
- T is the temperature in Kelvin.
Understanding Enthalpy Changes
Internal Energy (E) and Related Terms
- Energy: The capacity to do work or transfer heat; measured in joules (J).
- Internal Energy (E): Sum of all kinetic and potential energies within a system.
- ΔE = q + w (where q = heat, w = work)
- Heat (q): Energy transfer due to a temperature difference.
- Work (w): Energy transfer due to a force acting over a distance; for gases, w = -PΔV.
Quick Examples of Energy Transfer
- Heating Water on a Stove: Heat (q) flows into the pot, increasing water’s temperature.
- Lifting a Weight: Work (w) is done as a force moves the weight.
- Chemical Reactions in a Test Tube: Measure ΔH through the heat absorbed or released.
- Gas Compression: Work done on a gas increases its internal energy.
- Car Engines: Convert chemical energy from fuel into mechanical energy, with heat as a byproduct.
Practice Examples
1. Combustion of Methane (Exothermic)
Equation:
Description: ΔH is negative, indicating heat release.
2. Dissolution of Ammonium Nitrate (Endothermic)
Equation:
Description: ΔH is positive, indicating heat absorption.
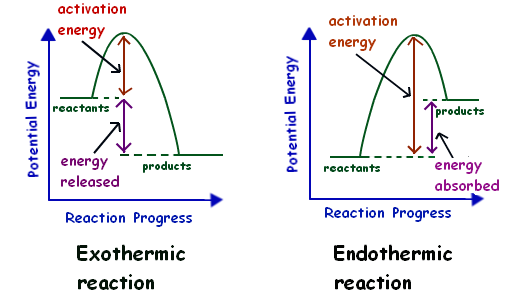

Image Courtesy of Mr Lowe
Additional Terms to Remember
- Energy (Joules): The ability to do work.
- Internal Energy (ΔE): Sum of all energies within a system.
- Heat (q): Energy transfer due to temperature difference.
- Work (w): Energy transfer due to force over a distance.
Summary
Enthalpy of reaction (ΔH) is a crucial measure in thermodynamics, indicating whether a reaction absorbs or releases heat. It helps predict reaction feasibility, understand energy transfers, and quantify heat changes. Mastering these concepts provides a strong foundation for tackling energy calculations and real-world applications in thermochemistry.
Recent Comments


6.6 Introduction to Enthalpy of Reaction


5.4 Conservation of Linear Momentum


5.3 Open and Closed Systems: Momentum

