Table of Contents
Toggle6.1 Endothermic and Exothermic Processes
Welcome to Thermochemistry! This section explores one of the most important concepts in chemistry: the energy changes that occur during chemical and physical processes. In this post, we’ll dive into the world of endothermic and exothermic reactions, helping you understand how energy flows, how to analyze different systems, and what makes certain reactions absorb or release heat.
Forms of Energy: A Quick Recap
In Unit 4, we discussed how matter undergoes both chemical and physical changes. While chemical changes involve forming or breaking ionic and covalent bonds between atoms, physical changes involve changes in the state of matter. These changes are closely tied to energy transfers, often seen as changes in temperature. For example, consider the melting of ice:
🔥 When ice melts, energy is supplied to convert it into liquid water. This leads us to three major types of energy relevant to thermochemistry:
1. Kinetic Energy
Kinetic energy is the energy of motion. The temperature of particles relates directly to their average kinetic energy; as temperature increases, particles move faster. This concept was introduced with the ideal gas law in Unit 3.
The formula for kinetic energy is:
- m is the mass (in kg)
- v is the velocity (in m/s)
- KE is expressed in Joules (J).
2. Potential Energy
Potential energy is stored energy due to an object’s position or state. In chemistry, it mainly refers to energy stored in chemical bonds between atoms and molecules. The stability of a compound is closely tied to its potential energy; more stable compounds have lower potential energy due to stronger, more stable bonds.
3. Electrostatic Energy
This is a form of potential energy resulting from the interactions between charged particles. It can be expressed using Coulomb’s Law:
- Q₁ and Q₂ are the charges.
- d is the distance between the charges.
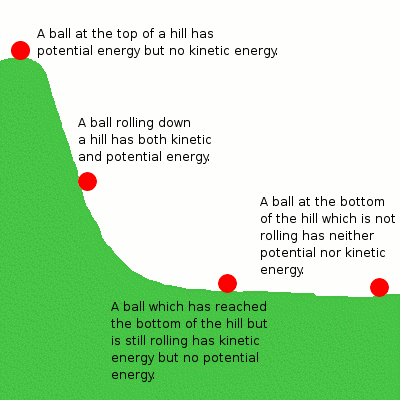

Image Courtesy of NRG
The Law of Conservation of Energy
The Law of Conservation of Energy states that energy cannot be created or destroyed; it can only be transferred or transformed. This fundamental law underpins all of thermodynamics and helps explain energy changes in chemical reactions.
For example, in a mechanical system like a ball rolling down a hill, potential energy converts into kinetic energy. Similarly, in chemical reactions, potential energy stored in bonds converts into heat energy or kinetic energy during the reaction.
This principle is also called the First Law of Thermodynamics.
Analyzing Energy Changes in Chemical Systems
Defining the System and Surroundings
- System: The specific part of the universe you’re studying, such as reactants and products in a reaction.
- Surroundings: Everything else outside the system.
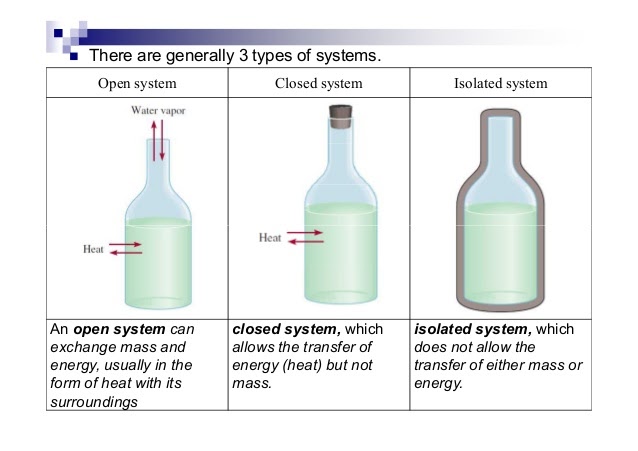
Types of Systems:
- Open System: Exchanges mass and energy with its surroundings (e.g., a pot of boiling water).
- Closed System: Exchanges energy but not mass (e.g., a sealed flask with a stopper).
- Isolated System: No exchange of mass or energy (e.g., an insulated calorimeter).
Image Courtesy of Socratic
State Functions
State functions describe the state of a system at any given time, regardless of how it got there. Examples include:
- Energy (E)
- Enthalpy (H)
- Pressure (P)
- Volume (V)
- Temperature (T)
Heat and Work Are Not State Functions
Heat and work depend on the pathway taken to reach a state. An analogy: the distance between two points (a state function) doesn’t change, but the route you take can vary.
Endothermic vs. Exothermic Processes
Endothermic Processes
Endothermic reactions absorb energy from their surroundings. In these processes:
- ΔH (change in enthalpy) is positive.
- Heat flows into the system.
Example: Melting ice is an endothermic process because heat flows into the ice, causing it to melt.
Exothermic Processes
Exothermic reactions release energy to their surroundings. In these processes:
- ΔH is negative.
- Heat flows out of the system.
Example: Burning wood is exothermic, as it releases heat and light.
Key Concept Recap:
- Endothermic: Heat enters the system (+ΔH).
- Exothermic: Heat leaves the system (-ΔH).
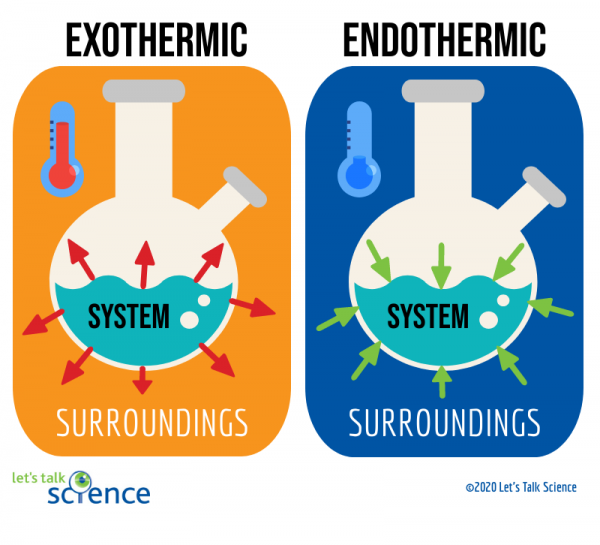

Image Courtesy of Let’s Talk Science
Real-Life Example: Hot and Cold Packs
Hot Packs contain substances like sodium acetate that release heat when activated (exothermic reaction).
Cold Packs use substances like ammonium nitrate that absorb heat (endothermic reaction), providing a cooling effect.
Explore More:
Mastering these concepts will help you excel in understanding energy transformations and thermochemistry for the AP Chemistry Exam. You’ve got this! 🌟


6.6 Introduction to Enthalpy of Reaction


3.8 Applications of Circular Motion and Gravitation


3.7 Free-Body Diagrams for Objects in Uniform Circular Motion


3.6 Centripetal Acceleration and Centripetal Force