

Table of Contents
ToggleMastering Reaction Mechanisms in AP Chemistry: A Guide to Rate-Determining Steps and Rate Laws
In previous guides, we explored how to find the rate law for reactions by analyzing experimental data. Now, it’s time to take things further by understanding reaction mechanisms—the actual step-by-step sequence of events leading from reactants to products. Mechanisms provide a window into the molecular interactions behind a reaction and help us determine the rate law when combined with experimental data.
What is a Reaction Mechanism?
A reaction mechanism breaks a chemical reaction into elementary steps that reveal how reactants transform into products. Unlike a simple balanced chemical equation, which only shows the overall transformation, a mechanism provides a detailed molecular pathway.
Key Concept: When you sum up all the elementary steps of a reaction, they must add up to the overall balanced equation.
Example Mechanism Breakdown
Consider the decomposition of ozone with iodide ions and water:
O3+2I−+H2O→O2+I2+2OH−
This mechanism consists of three elementary steps:
- Step 1 (Slow): O₃ + I⁻ → O₂ + IO⁻
- Step 2 (Fast): IO⁻ + H₂O → HOI + OH⁻
- Step 3 (Fast): HOI + I⁻ → I₂ + OH⁻
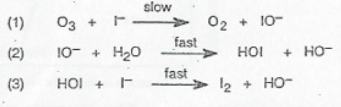

Analyzing the Mechanism:
- Intermediates: IO⁻ and HOI are intermediates—species formed during one step and consumed in another.
- Catalysts: A catalyst speeds up the reaction without being consumed. In this example, there is no catalyst present since no species reappear unchanged.
Understanding Rate-Determining Steps (RDS)
The rate-determining step is the slowest step in the mechanism, controlling the overall reaction rate. This concept is crucial because the rate law for the overall reaction is determined by the RDS.
Key Concept: The rate law of a reaction can be determined by using the stoichiometric coefficients of the reactants in the slow step (only for elementary steps). Outside of mechanisms, rate laws must be determined experimentally.
Writing a Rate Law Using a Mechanism
To find the rate law for a reaction mechanism, follow these steps:
- Identify the Rate-Determining Step: This is the slowest step.
- Write the Rate Law for the RDS: Use the stoichiometric coefficients as the exponents for reactant concentrations.
Example Free-Response Question Breakdown
Consider the decomposition of NO₂:
Given data shows that the reaction is second-order. We can confirm this by graphing 1/[NO₂] versus time and finding a linear relationship, indicating second-order kinetics.
Rate Law:
Now, let’s analyze two proposed mechanisms for this reaction:
Mechanism I
- Step 1 (Slow): 2 NO₂ → NO₃ + NO
- Step 2 (Fast): NO₃ + CO → NO₂ + CO₂
Is Mechanism I Consistent?
Since the slow step is the RDS, the rate law for this step is:
This matches the experimentally determined rate law, confirming that Mechanism I is consistent.
Mechanism II (with Intermediates)
- Step 1 (Fast Equilibrium): 2 NO₂ ⇌ N₂O₄
- Step 2 (Slow): N₂O₄ → 2 NO + O₂
Analyzing Mechanism II:
The slow step gives the rate law:
However, N₂O₄ is an intermediate and cannot appear in the final rate law. We use the equilibrium expression from Step 1:
Substituting this into the rate law:
This matches the experimental rate law, confirming that Mechanism II is also consistent.
Key Takeaways
- Mechanisms break reactions into elementary steps to show the detailed pathway from reactants to products.
- Rate-Determining Step (RDS) controls the overall reaction rate and determines the rate law.
- Intermediates must be replaced using equilibrium expressions when they appear in the RDS.
- Rate laws must be consistent with experimentally determined data for a proposed mechanism to be valid.
Final Thoughts
Understanding reaction mechanisms and rate-determining steps is crucial for mastering AP Chemistry kinetics. By breaking down complex reactions into manageable steps, you can uncover the true nature of chemical reactions and confidently write accurate rate laws.


5.10 Multistep Reaction Energy Profile


5.8 Reaction Mechanism and Rate Law


3.8 Applications of Circular Motion and Gravitation


3.7 Free-Body Diagrams for Objects in Uniform Circular Motion


3.6 Centripetal Acceleration and Centripetal Force

