

Table of Contents
ToggleUnderstanding Complex Reaction Mechanisms: Kinetics Beyond Elementary Steps
So far in our study of chemical kinetics, we’ve covered the basics of elementary reactions—those that occur in a single step and involve just one molecule or a group of atoms. However, real-world reactions are often much more complex, involving multiple steps and intricate mechanisms. Welcome to the fascinating world of reaction mechanisms and how they reveal the hidden steps of chemical reactions!
What is a Reaction Mechanism?
A reaction mechanism breaks down a complex reaction into its elementary steps, providing a detailed view of how reactants transform into products. While the net equation shows only the initial reactants and final products, the mechanism uncovers the individual steps that occur along the way.
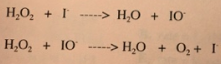
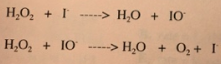
Example: Decomposition of Hydrogen Peroxide (H₂O₂)
Consider the decomposition of hydrogen peroxide:
- Step 1: H₂O₂ + I⁻ → H₂O + IO⁻
- Step 2: H₂O₂ + IO⁻ → H₂O + O₂ + I⁻
This reaction occurs in two elementary steps, with iodide (I⁻) playing a critical role. By adding the steps together and canceling out species that appear on both sides (e.g., I⁻ and IO⁻), we recover the overall reaction:
2H2O2→2H2O+O2
Catalysts and Intermediates
Iodide acts as a catalyst—it speeds up the reaction without being consumed. On the other hand, IO⁻ is an intermediate, formed during one step and consumed in the next. Recognizing catalysts and intermediates in mechanisms is essential for understanding reaction behavior and kinetics.

What a mechanism looks like for a more complex reaction. Luckily you won’t be dealing with these! Image Courtesy of ResearchGate
Mechanisms and Rate Laws: The Importance of Elementary Steps
Each elementary step in a mechanism has its own rate constant and activation energy. When analyzing complex reactions, the key is to identify the rate-determining step—the slowest step in the mechanism that controls the overall reaction rate.
Key Concept: Rate-Determining Step
The rate-determining step sets the pace for the entire reaction. Even if other steps are faster, the overall reaction can only proceed as fast as this slowest step. Conceptually, this makes sense: if you think of a multi-step reaction as an assembly line, the slowest worker determines the overall production speed.
Example: Finding the Rate Law for a Complex Mechanism
Let’s walk through an example to understand how to derive the rate law from a reaction mechanism.
Reaction Mechanism Example
Consider the two-step mechanism below:
- Step 1 (Slow): H₂ + ICl → HI + HCl
- Step 2 (Fast): HI + ICl → I₂ + HCl
Overall Reaction: H₂ + 2ICl → I₂ + 2HCl
Steps to Find the Rate Law:
Identify the Rate-Determining Step: Here, the slow step is the first step.
Write the Rate Law for the Slow Step:
R=k[H2][ICl]
Since we can use the stoichiometric coefficients for elementary steps, the rate law for the overall reaction is:This is because the rate of the overall reaction is determined by the slowest step.
When the Slow Step Involves an Intermediate
Sometimes, the rate-determining step may include an intermediate. Since intermediates don’t appear in the final rate law, we must substitute their concentrations using other steps (often involving equilibrium expressions). This substitution ensures that only reactants from the overall reaction appear in the rate law.
Practice Example: AP Chemistry 2019 Free-Response
Here’s a real-world example from the 2019 AP Chemistry exam:
Reaction Mechanism:
- Step 1 (Slow): NO₂ + NO₂ → NO₃ + NO
- Step 2 (Fast): NO₃ + CO → NO₂ + CO₂
Overall Reaction: NO₂ + CO → NO + CO₂
Finding the Rate Law:
- Identify the Slow Step: The first step is the slow step.
- Write the Rate Law: Using the stoichiometric coefficients for this elementary step, we have: R=k[NO2][NO2]=k[NO2]2
This shows how the overall reaction rate depends on the concentration of NO₂ squared.
Key Takeaways
- Elementary Steps: Break down complex reactions into simpler, single-step processes.
- Rate-Determining Step: The slowest step controls the overall reaction rate.
- Catalysts and Intermediates: Recognize their roles in reaction mechanisms.
- Rate Laws: For elementary steps, use stoichiometric coefficients; for complex reactions, focus on the slowest step.


5.10 Multistep Reaction Energy Profile


5.8 Reaction Mechanism and Rate Law


3.8 Applications of Circular Motion and Gravitation


3.7 Free-Body Diagrams for Objects in Uniform Circular Motion


3.6 Centripetal Acceleration and Centripetal Force

