

Table of Contents
ToggleUnderstanding the Collision Model in Chemical Kinetics
Chemical kinetics studies reaction rates—essentially, how quickly or slowly a chemical reaction proceeds. But what factors influence the speed of these reactions? Enter the collision model—a framework that explains how molecules must collide with the right energy and orientation for a reaction to occur.
What is the Collision Model?
The collision model depicts molecules as projectiles moving randomly with speeds dictated by temperature. When molecules collide, they may undergo a chemical reaction if two conditions are met:
- The collision must have sufficient energy to overcome the activation energy barrier.
- The molecules must be oriented correctly to break and form bonds, creating new products.
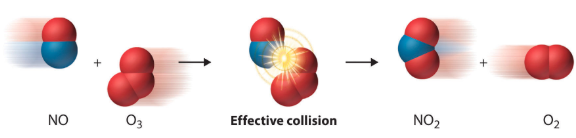

Image Courtesy of SaylorDotOrg
Visual Example: Nitrogen Monoxide and Ozone
Consider this reaction:
In this reaction, nitrogen monoxide and ozone collide effectively, leading to the formation of nitrogen dioxide and molecular oxygen. However, collisions must meet specific criteria to be successful.
Temperature and Molecular Speed
The rate of reaction depends significantly on the kinetic energy of the molecules, which is closely tied to temperature. As temperature increases, molecules move faster and collide more frequently with greater energy. This often accelerates reactions, a fact that’s commonly tested on the AP Chemistry exam.
Key takeaway: Raising the temperature generally increases the reaction rate due to higher kinetic energy and more frequent, energetic collisions.
The Math Behind the Collision Model (Optional Reading)
While the math isn’t required for the AP exam, understanding it conceptually deepens your grasp of the collision model. The model involves conservation of kinetic energy and momentum during molecular collisions. When molecules collide:
- Kinetic energy is partially converted to break chemical bonds and form new ones.
- Molecules move in various directions, leading to complex interactions within a sample.
For a more thorough exploration of the mathematical background, this section delves into statistical treatments of large numbers of molecules, emphasizing the relationship between average kinetic energy and temperature.
Interpreting the Collision Model
The collision model offers critical insights:
- Faster-moving molecules (more kinetic energy) increase the likelihood of effective collisions.
- Temperature plays a vital role—raising the temperature speeds up molecules, leading to more frequent and energetic collisions, which often results in a faster reaction rate.
Maxwell-Boltzmann Distributions
Maxwell-Boltzmann distributions describe how molecular energies vary within a sample at different temperatures. These graphs illustrate:
- Higher temperatures lead to a broader distribution of energies.
- A greater fraction of molecules have enough energy to exceed the activation energy barrier, increasing the rate of reaction.
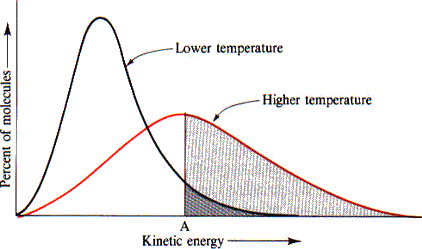

A Boltzmann Diagram showing the impact of higher temperatures, Image Courtesy of the University of Illinois at Urbana-Champaign
Effective vs. Ineffective Collisions
Not every collision leads to a chemical reaction. Effective collisions occur when molecules collide with sufficient energy and proper orientation to break existing bonds and form new ones. Conversely, ineffective collisions occur when:
- Molecules don’t have enough energy (e.g., they move too slowly).
- Molecules are improperly aligned, preventing bond formation.
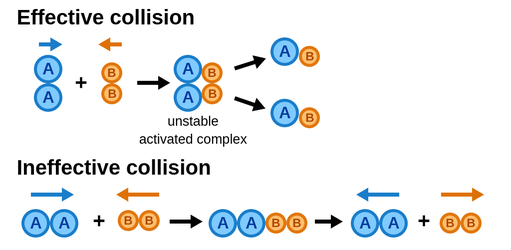

Image Courtesy of Labster Theory
Key Takeaways from the Collision Model
- Temperature influences molecular speed and energy. Higher temperatures increase reaction rates.
- Orientation and energy are critical—collisions must meet specific criteria to lead to a chemical reaction.
- Most collisions are ineffective; only a fraction result in a successful reaction.
Applying the Collision Model
Understanding the collision model helps explain why reactions proceed at different rates and how we can control reaction rates using temperature, catalysts, and concentration changes.
Recap:
- Effective collisions lead to reactions when molecules collide with enough energy and proper orientation.
- Increasing temperature raises kinetic energy, resulting in faster reactions.
By mastering the collision model, you’ll gain a better understanding of what drives chemical reactions and how to manipulate them—a vital skill in both academic and real-world chemistry applications.
Recent Comments


5.10 Multistep Reaction Energy Profile


5.8 Reaction Mechanism and Rate Law


5.4 Conservation of Linear Momentum


5.3 Open and Closed Systems: Momentum

