

Table of Contents
ToggleWriting and Understanding Rate Laws in Kinetics
In chemical kinetics, rate laws describe how the concentration of reactants affects the speed of a chemical reaction. Let’s explore how to find and write rate laws using experiments and practical examples to solidify your understanding.
What is a Rate Law?
A rate law expresses the relationship between the rate of a chemical reaction and the concentration of reactants. For a reaction A→B, the rate law can be expressed as:
R=k[A]n
where:
- R is the reaction rate.
- k is the rate constant.
- [A] is the concentration of reactant A.
- n is the order of the reaction with respect to A.
Key Points About Rate Laws
- Rate laws are determined experimentally: You cannot assume the order of a reaction based on stoichiometric coefficients.
- Reaction order (n) tells us how the concentration of a reactant impacts the reaction rate:
- If n = 1, doubling [A] doubles the rate (first order).
- If n = 2, doubling [A] quadruples the rate (second order).
- If n = 0, changes in [A] do not affect the rate (zero order).
Determining Rate Laws Experimentally
Since rate laws must be found experimentally, chemists conduct reactions at different reactant concentrations while keeping other conditions constant (especially temperature). This allows them to observe how the rate changes with varying concentrations.
Example Problem: Determining a Rate Law
Given the reaction:
2NO+2H2→N2+2H2O
and the data below, let’s find the rate law.
| Experiment | [NO] (M) | [H₂] (M) | Rate (mol/L·s) |
|---|---|---|---|
| 1 | 0.10 | 0.10 | 1.25 x 10⁻⁵ |
| 2 | 0.20 | 0.10 | 5.00 x 10⁻⁵ |
| 3 | 0.20 | 0.20 | 1.00 x 10⁻⁴ |
Step 1: Finding the Order with Respect to NO
Compare Experiments 1 and 2 where [H₂] remains constant:
- [NO] doubles, and the rate quadruples.
- Conclusion: The reaction is second order with respect to NO.
Step 2: Finding the Order with Respect to H₂
Compare Experiments 2 and 3 where [NO] remains constant:
- [H₂] doubles, and the rate doubles.
- Conclusion: The reaction is first order with respect to H₂.
Step 3: Writing the Rate Law
The rate law for this reaction is:
R=k[NO]2[H2]
To find k, plug in data from one of the experiments.
Understanding the Rate Constant (k)
k quantifies the speed of a reaction and varies with temperature. Its units depend on the reaction order:
- Zeroth-order: M/s
- First-order: s−1
- Second-order: M−1s−1
Elementary Reactions
An elementary reaction occurs in a single step and represents the basic process of reactants forming products. These reactions help us understand more complex reaction mechanisms. All rate law rules discussed here apply to elementary reactions.
AP Practice Question Example
Question: Given the decomposition of urea:
CO(NH2)2(aq)→NH4+(aq)+OCH−(aq)
at 90°C with the data below, determine if it is a first-order reaction.
| Time (hours) | [CO(NH₂)₂] (M) |
|---|---|
| 0 | 0.1000 |
| 5 | 0.0707 |
| 10 | 0.0500 |
| 15 | 0.0354 |
| 20 | 0.0250 |
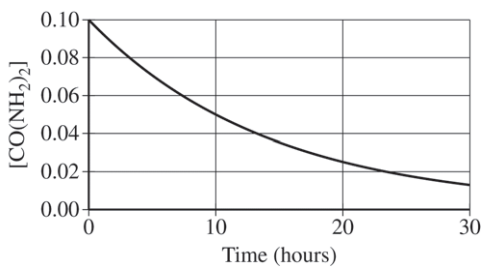

Solution:
- Identify constant half-life: The concentration halves every 10 hours, confirming first-order kinetics.
- Calculate the rate constant (k) using: t1/2=k0.693 With t1/2=10hours, we find k=0.0693h−1.
Experimental Setup to Test Rate Dependence on [OH−]
To test if the reaction rate depends on [OH⁻], conduct the reaction at different [OH⁻] concentrations while keeping other conditions constant and measure changes in [CO(NH₂)₂] over time.


5.10 Multistep Reaction Energy Profile


5.8 Reaction Mechanism and Rate Law


3.8 Applications of Circular Motion and Gravitation

3.7 Free-Body Diagrams for Objects in Uniform Circular Motion


3.6 Centripetal Acceleration and Centripetal Force

