

Table of Contents
ToggleUnderstanding Multistep Reactions: Constructing Reaction Energy Profiles and Analyzing Reactants, Intermediates, and Products
Introduction to Multistep Reactions
In many chemical reactions, the transformation of reactants into products does not occur in a single step. Instead, these reactions proceed through a series of intermediate stages called elementary reactions. When combined, these elementary reactions describe the overall process, which can be summarized by a chemical equation that displays the reactants, products, and their respective stoichiometric coefficients.
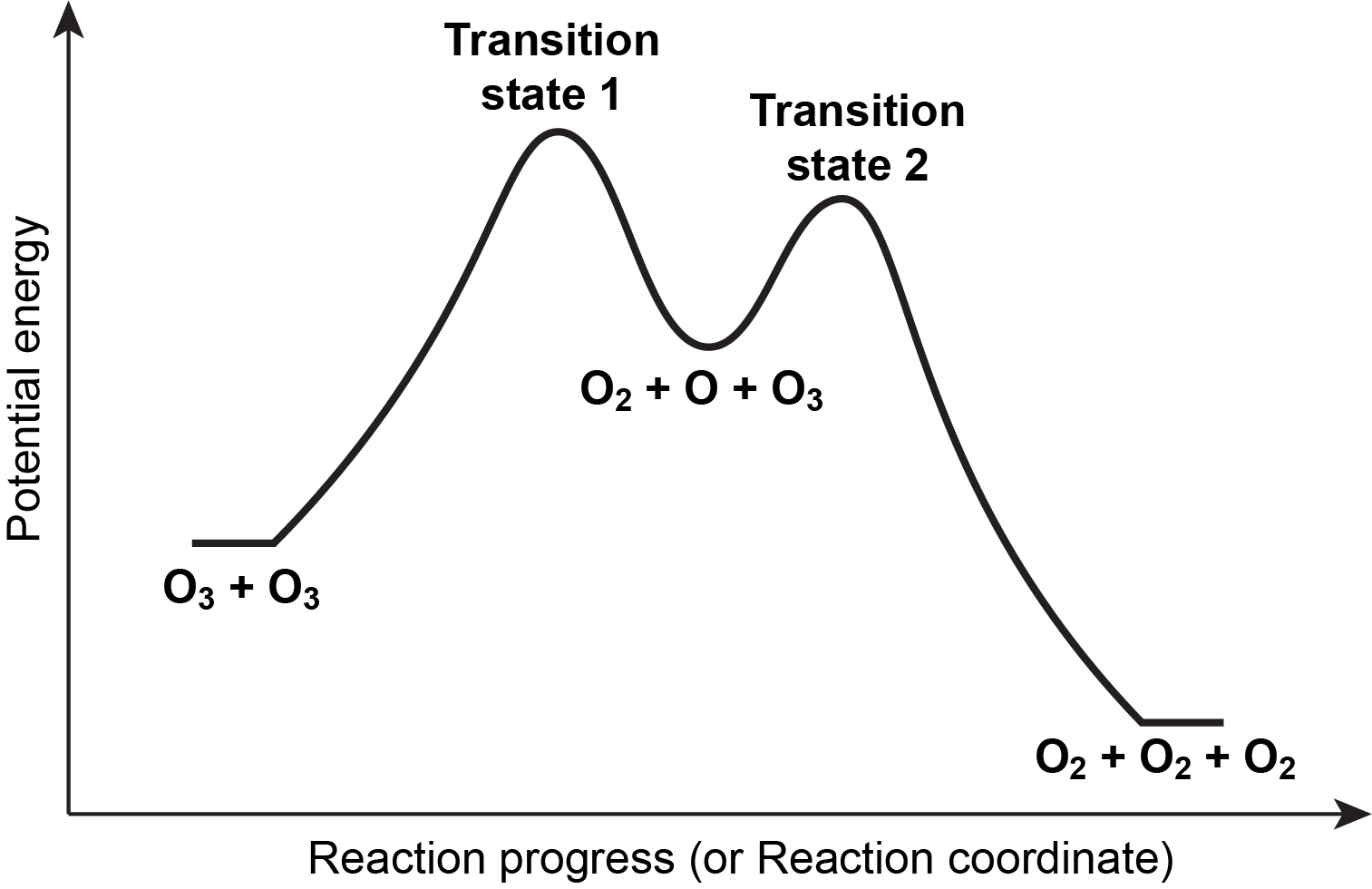

Source: University of Wisconsin
What is a Reaction Energy Profile?
A reaction energy profile visually represents the energy changes that occur during a chemical reaction, particularly in multistep reactions. It illustrates the activation energy, transition states, and overall energy change, giving a comprehensive picture of the energetics involved. This profile is crucial for understanding the rates and feasibility of reactions.
How to Construct a Reaction Energy Profile
Follow these steps to construct a reaction energy profile for a multistep reaction:
Plot the Reactants and Products:
Start by plotting the potential energy of the reactants on the left-hand side and the products on the right-hand side. The y-axis represents the potential energy, while the x-axis represents the progress of the reaction.Add Transition States:
Identify and mark the transition states along the reaction coordinate. These points represent the highest energy barriers that the reactants must overcome to be converted into products.Connect the Points:
Use curved lines to connect the reactants to the transition state(s) and the transition state(s) to the products. The curve represents the pathway the reaction follows and shows how energy changes during the process.Label Key Energies:
Clearly label the activation energy (ΔE1) and the overall energy change (ΔE2) on the graph. Activation energy is the energy required to reach the transition state from the reactants, while the overall energy change reflects the difference between the energy of the reactants and products.
Analyzing Reaction Energy Profiles: Endothermic vs. Exothermic Reactions
Endothermic Reactions:
In these reactions, the potential energy of the products is higher than that of the reactants. Energy is absorbed from the surroundings, resulting in an energy input to the system.
Example: Reactants + Energy→ProductsExothermic Reactions:
Here, the potential energy of the products is lower than that of the reactants. Energy is released into the surroundings, often as heat.
Example: Reactants→Products + Energy
Reactants, Intermediates, and Products
Understanding the roles of reactants, intermediates, and products is critical when analyzing reaction mechanisms:
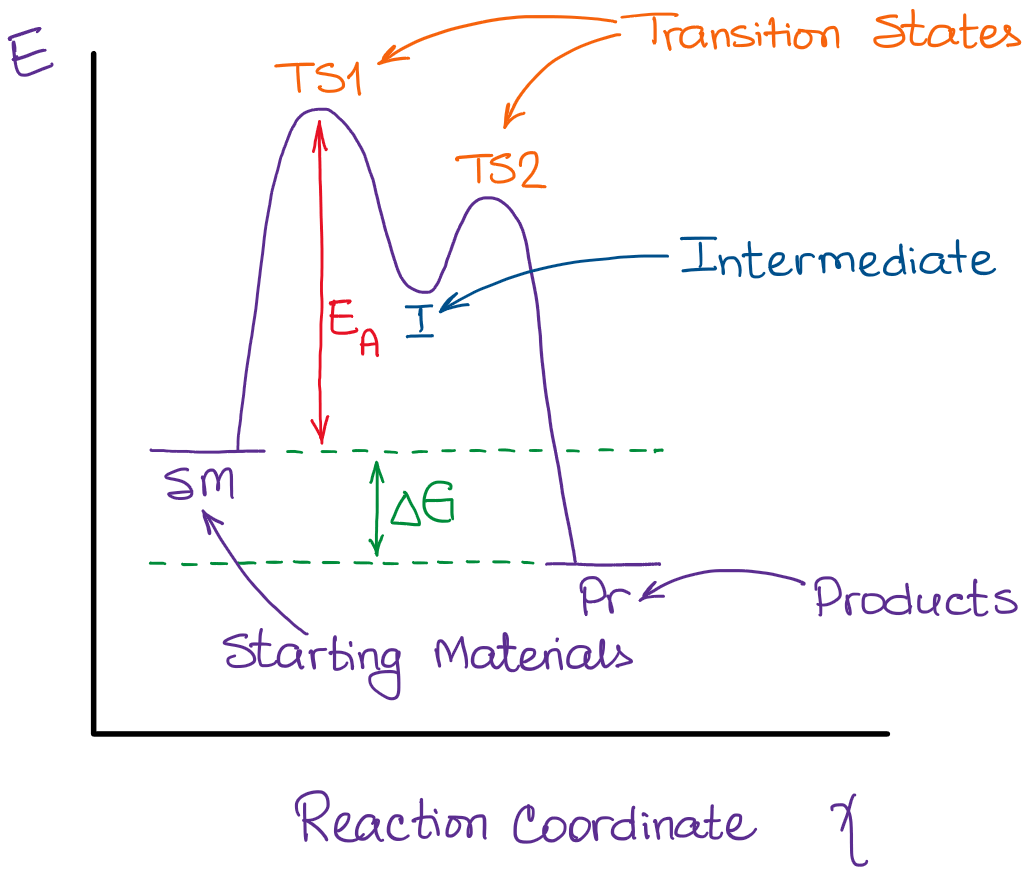

Reactants:
These are the starting substances that react to form new compounds. They appear on the left-hand side of a chemical equation.Intermediates:
Intermediates are species formed during the reaction but are consumed before the reaction completes. They are present only temporarily and do not appear in the overall balanced chemical equation.Products:
Products are the substances formed at the end of the reaction and are written on the right-hand side of a chemical equation.
Example Reaction: The Haber Process
The synthesis of ammonia (NH3) from hydrogen (H2) and nitrogen (N2) gases through the Haber process illustrates how reactants, intermediates, and products interact:
Reactants:
H2(g)+N2(g)Intermediate:
An intermediate, such as N2H3+, may form during the process.Products:
NH3(g)
The overall balanced equation for the Haber process is:
N2(g)+3H2(g)→2NH3(g)
Importance of Reaction Energy Profiles
Reaction energy profiles provide valuable insights into the activation energy required for a reaction, the stability of intermediates, and whether the reaction is endothermic or exothermic. This information is crucial for predicting reaction rates, understanding reaction mechanisms, and optimizing conditions for industrial and laboratory processes.
Key Takeaways
- Reaction Mechanisms: Break down complex reactions into elementary steps to better understand their behavior.
- Energy Profiles: Visualize the energy changes throughout a reaction to identify transition states and activation energies.
- Reactants, Intermediates, and Products: Clearly distinguish between these components to accurately describe and predict chemical behavior.
Conclusion
Mastering the construction and interpretation of reaction energy profiles provides a deeper understanding of the underlying energetics and kinetics of chemical reactions. By recognizing the roles of reactants, intermediates, and products, you can effectively analyze and predict the outcomes of complex reactions.


5.10 Multistep Reaction Energy Profile


5.8 Reaction Mechanism and Rate Law


3.8 Applications of Circular Motion and Gravitation


3.7 Free-Body Diagrams for Objects in Uniform Circular Motion


3.6 Centripetal Acceleration and Centripetal Force

