

Table of Contents
ToggleExploring Acid-Base Reactions: Understanding and Mastering Neutralization in Chemistry
Introduction
Welcome to the next chapter in mastering chemical reactions: acid-base neutralization reactions! In AP Chemistry, these reactions are fundamental and involve the transfer of a proton (H⁺) between molecules, leading to the formation of a salt and water. This guide delves into the Brønsted-Lowry definition, how to identify acids and bases, and how to write net ionic equations for neutralization reactions. Plus, we’ll tackle complex ion concentration problems for a well-rounded understanding.
Defining Acids and Bases: The Brønsted-Lowry Approach
The Brønsted-Lowry definition focuses on the transfer of protons (H⁺) between molecules:
- Acids are proton donors.
- Bases are proton acceptors.
But why is a proton equivalent to a hydrogen ion? 🤔
Understanding Protons and Hydrogen Ions
A proton is a positively charged subatomic particle in an atom’s nucleus. When a hydrogen atom (H) loses its electron, it becomes a hydrogen ion (H⁺)—essentially a lone proton. Therefore, in chemistry, proton and H⁺ are used interchangeably. You may also encounter H₃O⁺, the hydronium ion, as a hydrated form of H⁺ in aqueous solutions.
Identifying Acid-Base Reactions and Conjugate Pairs
In acid-base reactions, the transfer of H⁺ creates conjugate acid-base pairs. Here’s how to identify them:
Example Reaction: H₂O + H₂S → H₃O⁺ + HS⁻
Identify the pairs:
- Pair 1: H₂O and H₃O⁺
- Pair 2: H₂S and HS⁻
Classify acids and bases:
- H₂O gains a proton to become H₃O⁺ (conjugate acid), making H₂O the base.
- H₂S loses a proton to become HS⁻ (conjugate base), making H₂S the acid.
💡 Quick tip: The compound with the extra hydrogen is the acid in the pair!
Amphiprotic Substances: Dual Nature Explained
Amphiprotic substances can both donate and accept protons. A common example is water (H₂O), which can act as either an acid or a base, depending on the reaction context. NH₃⁻ is another amphiprotic substance.
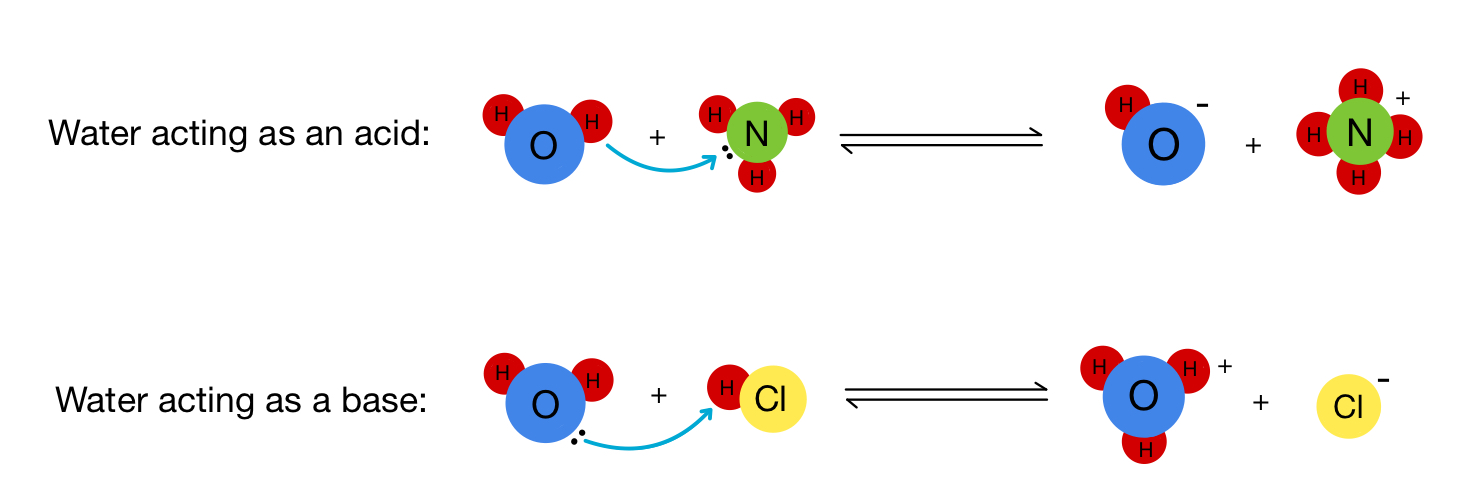

Image Courtesy of Expii
Acid-Base Neutralization: Reaction Fundamentals
In a neutralization reaction, an acid reacts with a base to form a salt and water:
General form: Acid + Base → Salt + Water
Example Reaction: HNO₃ (aq) + KOH (aq) → H₂O (l) + KNO₃ (aq)
Predicting products:
- H⁺ from HNO₃ combines with OH⁻ from KOH to form H₂O.
- Remaining ions (K⁺ and NO₃⁻) combine to form KNO₃.
Determining solubility:
- According to solubility rules, KNO₃ is soluble in water, so it remains aqueous.
Writing Net Ionic Equations for Acid-Base Reactions
Steps to Writing Net Ionic Equations
- Write the balanced chemical equation.
- Identify strong acids and bases (completely dissociate) versus weak acids and bases (partially dissociate).
- Dissociate strong acids/bases into ions.
- Remove spectator ions to write the net ionic equation.
Example: HNO₃ (aq) + KOH (aq) → H₂O (l) + KNO₃ (aq)
- Dissociate: H⁺ (aq) + NO₃⁻ (aq) + K⁺ (aq) + OH⁻ (aq) → H₂O (l) + K⁺ (aq) + NO₃⁻ (aq)
- Remove spectator ions (K⁺ and NO₃⁻): H⁺ (aq) + OH⁻ (aq) → H₂O (l)
Concentration of Ions in Acid-Base Reactions
Problem Example: Calculate the concentration of ions after neutralization. Given:
- 0.250 M and 28.0 mL of HNO₃
- 0.320 M and 53.0 mL of KOH
Calculate moles:
- HNO₃: 0.250 M×0.0280 L=0.00700 moles
- KOH: 0.320 M×0.0530 L=0.0170 moles
Identify limiting reactant (LR): HNO₃ (less moles).
Calculate excess reactant (OH⁻):
- Moles reacted: 0.00700 moles.
- Remaining moles: 0.0170−0.00700=0.010 moles
- Concentration: 0.010 moles/0.081 L=0.12 M
Final Answers:
- [H⁺] = 0
- [OH⁻] = 0.12 M
Practice Problem: Net Ionic Equation
Reaction: HNO₃ + Al(OH)₃
Steps:
- Write products: H₂O + Al(NO₃)₃.
- Balance: 3HNO₃ + Al(OH)₃ → 3H₂O + Al(NO₃)₃
- Identify states: 3HNO₃ (aq) + Al(OH)₃ (s) → 3H₂O (l) + Al(NO₃)₃ (aq)
- Dissociate aqueous substances: 3H⁺ (aq) + 3NO₃⁻ (aq) + Al(OH)₃ (s) → 3H₂O (l) + Al⁺³ (aq) + 3NO₃⁻ (aq)
- Remove spectator ions: 3H⁺ (aq) + Al(OH)₃ (s) → 3H₂O (l) + Al⁺³ (aq)
Conclusion
Mastering acid-base reactions requires understanding proton transfers, identifying conjugate pairs, and writing net ionic equations. With practice, these concepts become second nature, paving the way for deeper explorations in chemistry. Keep honing your skills, and you’ll be ready for any challenge that comes your way!
Recent Comments


4.9 Oxidation-Reduction (Redox) Reactions


4.8 Introduction to Acid-Base Reactions


4.7 Types of Chemical Reactions


5.2 Representations of Changes in Momentum

