

Table of Contents
ToggleReactions in Aqueous Solutions: Mastering Net Ionic Equations
Written by SlyAcademy Team
Table of Contents
- Introduction to Reactions in Aqueous Solutions
- The Dissolution of Ionic Compounds
- Understanding Precipitation Reactions
- Writing Net Ionic Equations
- General Steps for Writing Net Ionic Equations
- Example Problems for Practice
- Conclusion
Introduction to Reactions in Aqueous Solutions
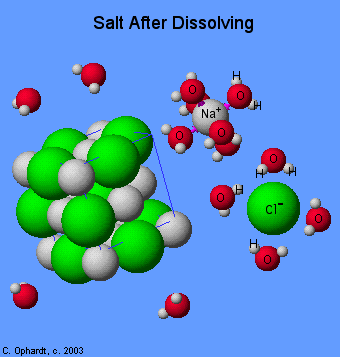
In AP Chemistry, most chemical reactions occur in aqueous solutions—meaning they take place in water. Water, being a polar molecule, dissociates ionic compounds into their constituent ions. When table salt (NaCl) dissolves, for example, water molecules surround and separate sodium (Na⁺) and chloride (Cl⁻) ions. This interaction occurs because water’s partially positive and negative ends are attracted to the oppositely charged ions, forming ion-dipole interactions.
Example Dissolution of NaCl:
NaCl (s) → Na⁺ (aq) + Cl⁻ (aq)
This dissociation is crucial for understanding how reactions in aqueous solutions proceed, as it’s often the ions themselves—rather than whole molecules—that react.
The Dissolution of Ionic Compounds
Ionic compounds dissociate into ions when dissolved in water. For example, NaCl dissociates into Na⁺ and Cl⁻ ions due to the polar nature of water. This concept ties directly to intermolecular forces. In solutions, you’ll see ion-dipole interactions: the positive end of the water molecule (hydrogen) interacts with the negative chloride ion, while the negative end of the water molecule (oxygen) attracts the positive sodium ion.

Understanding Precipitation Reactions
Precipitation reactions occur when two solutions combine and form an insoluble product—known as a precipitate—that falls out of solution. Not all compounds dissolve in water; insoluble compounds form solids instead.
Example of Precipitation Reaction:
2KI (aq) + Pb(NO₃)₂ (aq) → 2KNO₃ (aq) + PbI₂ (s)
Lead (II) iodide is insoluble, so it precipitates as a solid. Understanding solubility rules is key to predicting whether a precipitate will form. While you don’t need to memorize every rule for the AP exam, remember that all sodium, potassium, ammonium, and nitrate salts are soluble in water.
Image Courtesy of Regents Chemistry Reference Table
Writing Net Ionic Equations
A net ionic equation shows only the species directly involved in the reaction, omitting spectator ions that don’t participate in forming the product. This provides a clearer view of the chemistry happening at the ionic level.
Example 1: Precipitation Reaction
Reaction:
2KI (aq) + Pb(NO₃)₂ (aq) → 2KNO₃ (aq) + PbI₂ (s)
Write the Complete Ionic Equation:
2K⁺ (aq) + 2I⁻ (aq) + Pb²⁺ (aq) + 2NO₃⁻ (aq) → 2K⁺ (aq) + 2NO₃⁻ (aq) + PbI₂ (s)
Only soluble compounds are dissociated into ions. PbI₂ is insoluble and remains as a solid.Identify and Remove Spectator Ions:
Spectator ions are ions that appear unchanged on both sides of the equation (in this case, K⁺ and NO₃⁻).Net Ionic Equation:
2I⁻ (aq) + Pb²⁺ (aq) → PbI₂ (s)
This equation shows the formation of the solid PbI₂ from its constituent ions.
General Steps for Writing Net Ionic Equations
Follow these steps to write a net ionic equation:
- Identify Soluble and Insoluble Compounds: Use solubility rules to determine which compounds dissociate in water.
- Balance the Chemical Equation: Ensure the reaction is balanced.
- Write the Complete Ionic Equation: Dissociate all soluble compounds into ions. Leave insoluble compounds intact.
- Omit Spectator Ions: Remove ions that appear unchanged on both sides of the equation.
- Write the Net Ionic Equation: Focus on the ions and molecules that participate in the reaction.
Example Problems for Practice
Example 2:
Reaction:
KOH (aq) + Fe(NO₃)₃ (aq) → KNO₃ (aq) + Fe(OH)₃ (s)
- Determine Solubility:
- Fe(OH)₃ is insoluble and forms a precipitate.
- Balance the Equation:
3KOH (aq) + Fe(NO₃)₃ (aq) → 3KNO₃ (aq) + Fe(OH)₃ (s) - Complete Ionic Equation:
3K⁺ (aq) + 3OH⁻ (aq) + Fe³⁺ (aq) + 3NO₃⁻ (aq) → 3K⁺ (aq) + 3NO₃⁻ (aq) + Fe(OH)₃ (s) - Identify Spectator Ions:
K⁺ and NO₃⁻ are spectator ions. - Net Ionic Equation:
3OH⁻ (aq) + Fe³⁺ (aq) → Fe(OH)₃ (s)
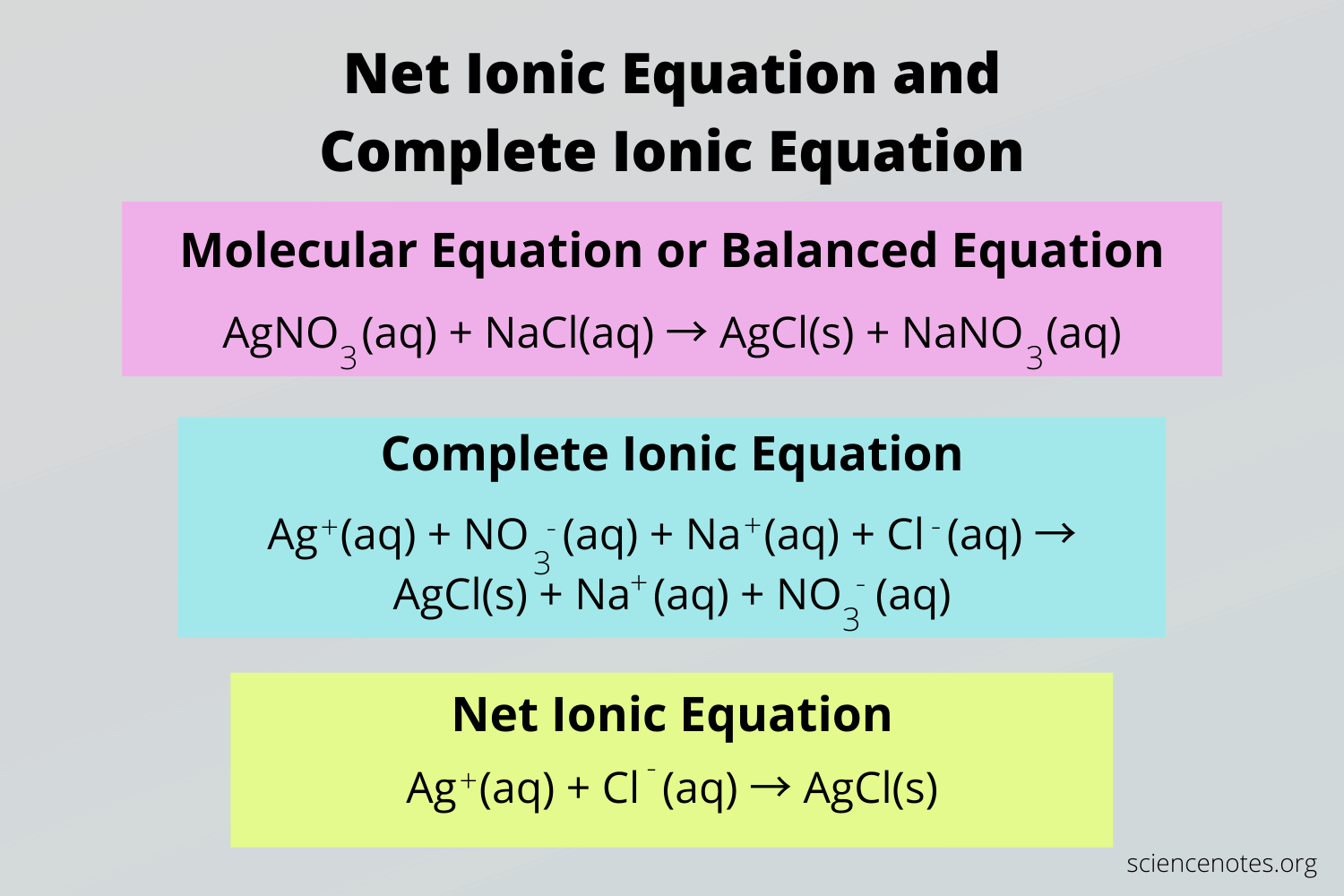

Image Courtesy of Science Notes
Conclusion
Mastering reactions in aqueous solutions and writing net ionic equations is a key skill in AP Chemistry. By focusing on the ions that actively participate in reactions, you gain deeper insight into the processes at play. Practice these skills, use solubility rules wisely, and you’ll excel in this foundational aspect of chemistry.
Recent Comments


4.9 Oxidation-Reduction (Redox) Reactions


4.8 Introduction to Acid-Base Reactions


4.7 Types of Chemical Reactions


5.2 Representations of Changes in Momentum

