3.3 Solids, Liquids, and Gases


Table of Contents
Toggle3.3 States of Matter
In Unit 1, we learned about matter, which is any physical object that has mass and occupies space. Matter can be classified by its state (solid, liquid, gas) or its composition (atoms and molecules). In this unit, we’ll focus on gases and solutions, but first, let’s review the characteristic properties of the states of matter.
🧊 Solids
Solids can either be crystalline or amorphous:
- Crystalline solids have a well-ordered, 3D structure.
- Amorphous solids lack long-range order.
Regardless of type, solids:
- Retain their shape and volume.
- Don’t expand to fill their container.
- Have strong intermolecular forces that keep particles closely packed and relatively immobile. However, the particles do vibrate!
- Are incompressible, don’t flow, and have slow diffusion.
💧 Liquids
Liquids:
- Assume the shape of the container they occupy, but do not expand to fill the container.
- Have particles that can flow past one another (fluidity) due to weaker intermolecular forces than solids.
- Are virtually incompressible, flow readily, and diffusion occurs more quickly than in solids.
The solid and liquid phases of a substance typically have similar molar masses, as their particles are closely packed together.
Surface Tension
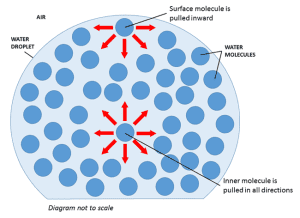
Surface tension is the tendency of liquids to minimize their surface area due to an imbalance of intermolecular forces. Molecules on the surface experience a net inward force. This causes liquids to form spherical droplets to reduce surface area.
Trends with surface tension:
- Stronger intermolecular forces → higher surface tension (surface molecules resist penetration).
- Higher temperature → lower surface tension (particles move more, making it easier to stretch the surface).
Capillary Action
Capillary action is the spontaneous rising of a liquid against gravity (often seen with polar liquids with strong IMFs). You can observe this when a paper towel absorbs water from a puddle.
There are two types of forces involved:
- Cohesive forces: Attraction between liquid molecules.
- Adhesive forces: Attraction between liquid molecules and the container.
Capillary action occurs when adhesive forces pull surface molecules upward and cohesive forces pull bulk molecules upward with them. These two forces work together to make this happen.
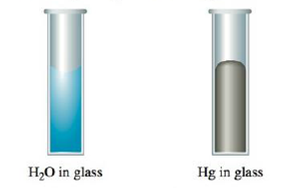

Meniscus: When you see a concave meniscus (e.g., water), it’s because adhesive forces are stronger than cohesive forces. Conversely, a convex meniscus (e.g., mercury) happens when cohesive forces are stronger than adhesive forces.
Viscosity
Viscosity is a measure of a liquid’s resistance to flow. The stronger the intermolecular forces, the higher the viscosity (the thicker the liquid).
Trends with viscosity:
- Stronger IMFs → higher viscosity (think of syrup).
- Higher temperature → lower viscosity (as higher energy allows particles to overcome IMFs more easily).
♨️ Gases
Gases:
- Assume the volume and shape of their container.
- Gas particles move rapidly and freely, overcoming any intermolecular forces.
- Compressible, flow readily, and diffusion occurs quickly.
Density
Density measures how compact a substance is. The formula is D = m/V (density equals mass divided by volume).
Solids are generally the most dense, while gases are the least dense due to the space between their particles.
Density Practice Question
A student measured the mass of a sealed 644 mL flask containing air. They flushed the flask with an unknown gas and resealed it, then measured the mass again. Calculate the mass of the unknown gas. The density of air at STP is 1.29 g/L.
| Volume of Sealed Flask | 644 mL |
|---|---|
| Mass of Sealed Flask and Air | 121.03 g |
| Mass of Sealed Flask and Unknown Gas | 122.60 g |
Step-by-Step Solution
Step 1: Find the mass of the air
We know the density of air is 1.29 g/L and the volume of the flask is 644 mL (0.644 L). Use the formula D = m/V to find the mass of the air:
D = m/V → 1.29 = m / 0.644 → m = 0.831 g (mass of air)
Step 2: Find the mass of the flask
Now, subtract the mass of the air from the total mass of the sealed flask with air:
121.03 g – 0.831 g = 120.20 g (mass of the empty flask)
Step 3: Find the mass of the unknown gas
Subtract the mass of the flask from the total mass of the flask with the unknown gas:
122.60 g – 120.20 g = 2.40 g (mass of the unknown gas)
Summary of States of Matter
Here’s a summary chart comparing the properties of solids, liquids, and gases:
| Property | Solids | Liquids | Gases |
|---|---|---|---|
| Shape | Fixed | Takes shape of container (partially) | Takes shape of container (entirely) |
| Volume | Fixed | Fixed | Takes volume of container |
| Compressibility | Virtually incompressible | Virtually incompressible | Highly compressible |
| Flow | Does not flow | Flows readily | Flows readily |
| Diffusion | Very slow | Moderate | Rapid |
| IMFs | Strong | Moderate | Negligible |
Understanding the states of matter is essential for learning about gases and solutions later in this unit. Be sure to familiarize yourself with the unique properties of solids, liquids, and gases, and how these relate to intermolecular forces and density.


3.11 Spectroscopy and the Electromagnetic Spectrum


Discover the Ultimate Multiplication Tables Generator – A Comprehensive Guide


Basic Algebra Concepts: Lesson 2.2