
Table of Contents
ToggleQuantum Mechanics: Where is the Electron?
Early models of the atom attempted to explain atomic structure. The first model was the Dalton Model, which represented atoms as small, indivisible, and indestructible spheres. But as more discoveries were made, scientists realized atoms weren’t so simple.
Next came Thomson’s Plum Pudding Model after J.J. Thomson discovered the electron. This model proposed that an atom consisted of a “sea” of positive charge with negatively charged electrons scattered throughout, like raisins in a plum pudding. 🍮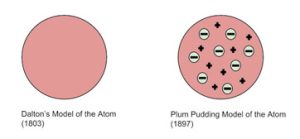

Rutherford’s Gold Foil Experiment followed, where alpha particles were fired at a thin sheet of gold. Most of the particles passed through, but some deflected sharply. This led Rutherford to conclude that the atom’s positive charge was concentrated in a small, dense nucleus at the center of the atom, with electrons surrounding it in mostly empty space.
Big Question: These models led to the fundamental question that created quantum mechanics: Where do the electrons go? Understanding this required diving into the nature of light and energy, leading to the field of quantum mechanics.
Max Planck and the Ultraviolet Catastrophe
Scientists observed that when metals were heated, they emitted light. This observation led to the concept of the blackbody, an object that absorbs all radiation. However, according to classical theory, blackbodies should emit infinite amounts of energy at high frequencies—an impossibility known as the Ultraviolet Catastrophe. 🌌
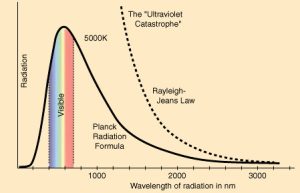
Max Planck’s Solution: In 1900, Max Planck proposed that light is emitted in discrete packets, or quanta, where the energy is proportional to the frequency of the light wave. This relationship is expressed by the equation:
where E is energy, ν is frequency, and h is Planck’s constant ( .
This discovery laid the foundation for quantum mechanics.
The Photoelectric Effect
Albert Einstein built on Planck’s work by explaining the Photoelectric Effect, where electrons are ejected from a metal surface when exposed to light of a certain minimum frequency. 🧐 This effect puzzled scientists because they expected the number of electrons ejected to depend on the light’s intensity, not its frequency. Einstein proposed that light behaves as both a wave and a particle (photon), where each photon carries energy proportional to its frequency.
The Photoelectric Effect demonstrated that when light reaches a certain threshold frequency, it has enough energy to eject electrons from the surface of a metal. This led to the equation:
where hv is the energy of the photon, KE is the kinetic energy of the ejected electron, and the binding energy is the energy required to release the electron.
Key points to remember:
- If the frequency of light is too low, it will not eject electrons.
- When the frequency is high enough, electrons are ejected, and the kinetic energy of these electrons increases as the frequency increases.


The Speed of Light and Light Equations
There are two key equations to remember:
- Energy equation (Planck’s Equation):
- Speed of light equation:
where c is the speed of light , λ is the wavelength, and ν is the frequency.
These equations are often used together to calculate the energy of photons and the behavior of electrons in the context of quantum mechanics.


3.11 Spectroscopy and the Electromagnetic Spectrum


Discover the Ultimate Multiplication Tables Generator – A Comprehensive Guide


Basic Algebra Concepts: Lesson 2.2