Table of Contents
Toggle3.1 Intermolecular Forces (IMFs)
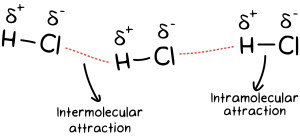
Intermolecular forces (IMFs) are attractive or repulsive forces between molecules due to differences in charge. Many students often confuse IMFs with intramolecular forces, so it’s important to remember the distinction:
- Intermolecular forces: Forces between molecules.
- Examples: London dispersion forces, dipole-dipole forces, hydrogen bonds, ion-dipole forces
- Intramolecular forces: Forces within a molecule that hold atoms together.
- Examples: Covalent bonds, metallic bonds, ionic bonds
A helpful tip: think “inter” = between molecules and “intra” = within a molecule.
IMFs are much weaker than intramolecular forces because they occur over larger distances. According to Coulomb’s Law, the closer two particles are, the stronger their attraction.

In this guide, we’ll learn about four types of IMFs: London dispersion forces, dipole-dipole forces, hydrogen bonding, and ion-dipole forces.
London Dispersion Forces (LDFs)
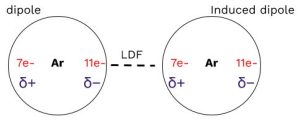
London Dispersion Forces (LDFs) are the weakest type of IMF and exist in all molecular samples, including nonpolar molecules and noble gases. On the AP Exam, if you’re asked about the types of IMFs present in a molecule, LDFs will always be one of the answers!
LDFs occur because electrons in a molecule can randomly shift, creating a temporary dipole. This temporary dipole can then induce dipoles in neighboring molecules, resulting in attractive forces between the partially negative and positive sides of the molecules.
Key point: The strength of LDFs increases as the size of a molecule increases. More electrons lead to a more polarizable electron cloud, which is easier to distort and form temporary dipoles.

Dipole-Dipole Interactions
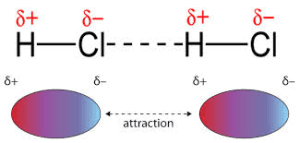
Dipole-dipole interactions occur between the opposite partial charges of permanent dipoles in polar molecules. These forces are stronger than LDFs, as the molecules have permanent dipoles (instead of temporary dipoles like LDFs).
The strength of the dipole-dipole interaction increases as the polarity of the molecules increases. The higher the difference in electronegativity between the atoms, the greater the dipole and the stronger the force.

AP Question – LDFs + Dipole-Dipole
2018 AP Chemistry Exam:
Question: CS₂ and COS both have LDFs, but COS also has dipole-dipole interactions. Explain why CS₂ has a higher boiling point.
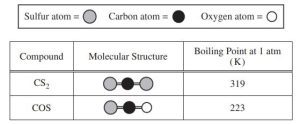

Sample Response: Both CS₂ and COS have LDFs, but COS also has dipole-dipole forces. However, the LDFs in CS₂ are stronger due to its larger size, outweighing the dipole-dipole forces in COS, resulting in a higher boiling point for CS₂.
Key Takeaway: Even though LDFs are generally weak, in large molecules, they can be stronger than dipole-dipole forces.
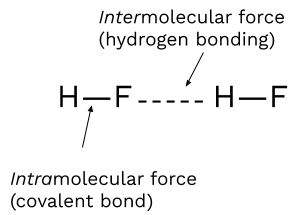
Hydrogen Bonding
Hydrogen bonding is a particularly strong type of dipole-dipole attraction that occurs when hydrogen is directly bonded to fluorine (F), oxygen (O), or nitrogen (N). Despite its name, hydrogen bonding is not a true bond but rather a strong IMF.
A good way to remember hydrogen bonding is: “Hydrogen bonding is FON.”
Hydrogen bonds lead to higher boiling points due to the strong attraction between molecules, as seen in water (H₂O). This is why water has such a high boiling point relative to its molecular size.
Hydrogen bonding also plays a crucial role in DNA structure.

Ion-Dipole Forces
Ion-dipole forces occur when an ion (either a cation or anion) interacts with the partial charges of a polar molecule. These forces are much stronger than dipole-dipole interactions or hydrogen bonds.
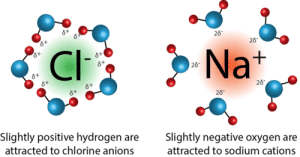

For example, when NaCl dissolves in water, the sodium cations (Na⁺) are attracted to the oxygen (partial negative charge) of water, while the chloride anions (Cl⁻) are attracted to the hydrogen (partial positive charge) of water.
Ion-Ion Attractions
Ion-ion attractions occur in ionic compounds and are the strongest type of IMF. These attractions exist between fully charged ions (e.g., Na⁺ and Cl⁻) in a crystal lattice. This is why ionic compounds, like NaCl, have such high melting and boiling points.
Ion-ion forces should not be confused with ion-dipole forces.
- Ion-ion forces occur between ions within a solid.
- Ion-dipole forces occur between ions and polar molecules in a solution.
Summary of Intermolecular Forces
You can determine the dominant IMF using the following order of strength:
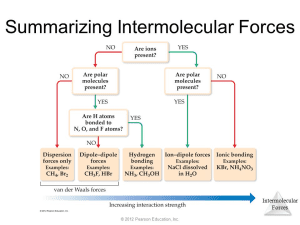

- Ion-ion interactions (strongest)
- Ion-dipole interactions
- Hydrogen bonding
- Dipole-dipole interactions
- London dispersion forces (LDFs) (weakest)


3.11 Spectroscopy and the Electromagnetic Spectrum


Discover the Ultimate Multiplication Tables Generator – A Comprehensive Guide


Basic Algebra Concepts: Lesson 2.2