2.6 Resonance and Formal Charge


Table of Contents
ToggleResonance Structures
Sometimes there isn’t just one way to represent a molecule using a Lewis dot structure (LDS). When multiple valid Lewis structures can be drawn, the molecule exhibits resonance. Think of resonance as similar to mixing paint 🎨—the molecule is a blend, or average, of all possible resonance structures.
What is Resonance?
- Resonance occurs when a molecule can be represented in multiple ways using valid Lewis structures.
- The actual structure is an average of these resonance forms. This often leads to fractional bond orders (like 4/3 or 2/3), where the bonds in reality are neither purely single nor purely double bonds.
A common misconception is that resonance means the molecule actually switches between these forms, but in reality, the true structure is a hybrid—a mix of all possible resonance forms.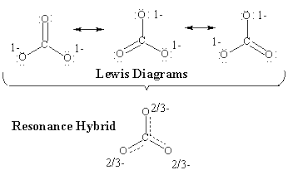
How to Identify Resonance
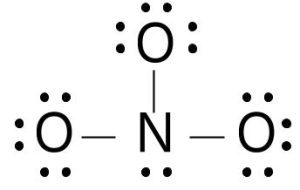
Let’s take a look at how to draw the Lewis structure of the polyatomic ion NO₃⁻ (nitrate):
Count valence electrons: Nitrogen (N) has 5 valence electrons, and each oxygen (O) atom has 6.
- Total: 5 + (3 × 6) = 23
- Since NO₃⁻ has a -1 charge, add 1 more electron for a total of 24 valence electrons.
Draw the central atom: Nitrogen is the central atom, surrounded by 3 oxygen atoms.
Draw single bonds between nitrogen and each oxygen, giving each oxygen a full octet. Initially, this gives us 26 electrons, but we only have 24! To fix this, replace a single bond with a double bond.
Draw resonance structures: You can place the double bond in three possible places, creating three resonance structures.

Resonance in Reality
In reality, the nitrate ion doesn’t have two single bonds and one double bond. Instead, all three bonds are equal, with a bond order of 4/3 (since there are 4 bonding electrons spread across 3 spaces).
To calculate bond order, use this formula:
Bond order = (# of bonds / # of bond spaces)
In this case, we have 4 bonds spread across 3 spaces, leading to a bond order of 4/3.
Formal Charge
Formal charge is used to determine the best Lewis structure by calculating the charge on each atom, assuming equal sharing of electrons. This helps predict the most stable arrangement of electrons.
How to Calculate Formal Charge
The formula for formal charge is:
Formal charge = (# of valence electrons) – (# of dots) – (# of dashes)
For example, in the SCN⁻ ion (thiocyanate):
- Sulfur has 6 valence electrons, 2 lone electrons (dots), and 3 bonds (dashes).
Formal charge = 6 – 2 – 3 = +1
Example: Formal Charge for Phosphate (PO₄³⁻)
Count valence electrons:
- P: 5 valence electrons
- O: 6 valence electrons each (×4)
- Total: 32 electrons, including the -3 charge.
Draw phosphorus in the center with four single bonds to oxygen atoms. This gives phosphorus a formal charge of +1 and each oxygen a formal charge of -1.
To minimize the formal charge, add a double bond to one of the oxygen atoms.
Now, phosphorus has a formal charge of 0, and one oxygen also has a formal charge of 0, making the structure more stable.
Quick Tip: The sum of formal charges should equal the charge of the ion. In this case, the sum is -3, which matches the charge of PO₄³⁻.
AP Chemistry Practice
Practice Question 1 (AP Exam 2016)
HCO₃⁻ ion: This ion has three C-O bonds, two of which are the same length and one that is longer. You’re asked to draw the Lewis structure.
- Calculate the total valence electrons (24 electrons) and draw carbon as the central atom. Attach the oxygen atoms and hydrogen to one oxygen.
- Two C-O bonds are single, and one is a double bond, resulting in resonance.
You would need to draw all resonance structures for full credit, using double-headed arrows (↔️) between the structures.
Practice Question 2 (AP Exam 2017)
S₂Cl₂: Complete the Lewis dot diagram by drawing all electron pairs.
- S₂Cl₂ consists of sulfur-sulfur and sulfur-chlorine single bonds. Don’t forget the lone pairs on each chlorine atom.
Practice Question 3 (AP Exam 2017)
Two possible Lewis structures for HCNO (fulminic acid) are given, and you are asked to explain which is better based on formal charge.
- The best structure is the one where oxygen (the most electronegative atom) has a negative formal charge. Formal charge calculations show that the diagram with oxygen having a -1 charge is better.
Summary of Key Concepts
Resonance: Molecules can be represented by multiple Lewis structures, and the true structure is an average of these forms.
Formal Charge: Helps determine the most stable structure by assigning charges based on electron sharing.
Bond Order: Indicates the strength of bonds; resonance structures often result in fractional bond orders.
Try It Yourself!
- Draw the Lewis structure for NO₂⁻ (nitrite ion). How many resonance structures can you draw?
- Calculate the formal charge of the atoms in SO₄²⁻ (sulfate ion). How can you adjust the structure to minimize formal charges?
Recent Comments





