2.3 Structure of Ionic Solids


Table of Contents
ToggleAtoms and Ionic Solids
Atoms come together to form molecules, right? But what about ionic solids? In ionic solids, the specific atoms involved are referred to as cations (positive ions) and anions (negative ions). The careful use of these terms is crucial for your Free Response Question (FRQ) answers, so always use the correct language!
- Cation: A positively charged ion, typically a metal.
- Anion: A negatively charged ion, typically a nonmetal.
Ionic bonding usually occurs when a metal loses a valence electron to a nonmetal. This electron transfer makes the metal a cation and the nonmetal an anion.
These oppositely charged ions interact because of their opposite charges, and their interaction is described by Coulomb’s Law.
Structure of Ionic Solids
Ionic solids are often brittle and hard, with high melting points. This results from their ions being arranged in a 3D crystal lattice, where each positive ion is surrounded by negative ions and vice versa.
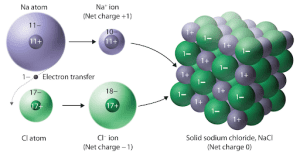

- Crystal lattice: A repeating 3D arrangement of cations and anions to maximize attractive forces while minimizing repulsive forces.
As a result of ionization:
- Cations (metal ions) become smaller when they lose electrons.
- Anions (nonmetal ions) become larger when they gain electrons.
In the example of Na⁺ and Cl⁻, the small sodium cations fit between the larger chloride anions in the lattice, enhancing the ionic attraction.
Representation of Ionic Solids
Ionic solids are typically represented by a network of positive and negative ions (unlike molecular substances with covalent bonds, represented by discrete molecules like H₂O). Ionic diagrams show this network clearly.
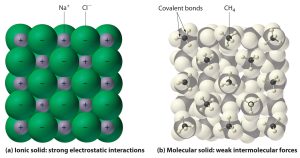
Explaining the Lattice Structure
The lattice structure in ionic solids is explained by the strong electrostatic forces between cations and anions, as described by Coulomb’s Law:
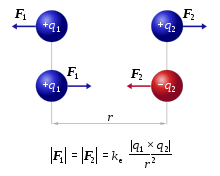

- Magnitude of charge: The greater the charge on the ions, the stronger the attraction.
- Distance between ions: The closer the cations and anions, the stronger the attraction.
In a structure like NaCl, small sodium cations fit closely between the chloride anions, resulting in strong electrostatic forces.
Properties of Ionic Substances
Ionic substances generally have the following characteristics:
🌡️ High melting and boiling points: The strong electrostatic forces require lots of energy to overcome.
🚫🔌 Poor conductors of electricity as solids: Electrons are localized and unable to move in the solid state, preventing electrical conductivity.
⚡ Good conductors in liquid or aqueous states: When melted or dissolved, ions can move freely, allowing the substance to conduct electricity.
💎 Hard and brittle: The strong electrostatic forces make ionic solids hard and difficult to deform.
Lattice Energy
Lattice Energy is the energy released when ions form an ionic solid. This is another place where Coulomb’s Law is relevant:
- The smaller the ions and the greater the charge, the higher the lattice energy and the higher the melting point of the ionic solid.
Review of Coulomb’s Law:
Let’s recall how to determine which ionic compound has a higher melting point. For example:
NaF vs. NaCl
- Both have the same charge (+1/-1), so we compare their ion sizes.
- F⁻ is smaller than Cl⁻, so the Na⁺-F⁻ bond is stronger, and NaF has the higher melting point.
Examples: Determining Lattice Energy
Lattice energy depends on charge and distance (Coulomb’s Law). Let’s apply this:
NaF or NaCl?
- NaF has the higher lattice energy because F⁻ is smaller than Cl⁻.
MgO or NaF?
- MgO has a higher lattice energy because Mg²⁺ and O²⁻ have higher charges (+2/-2) compared to Na⁺ and F⁻ (+1/-1).
NaF or KCl?
- NaF has higher lattice energy because Na⁺ and F⁻ are smaller than K⁺ and Cl⁻.
LiCl or NaCl?
- LiCl has higher lattice energy because Li⁺ is smaller than Na⁺.
Check Your Understanding: Advanced Practice
The following question is from the Advanced Placement YT Channel. Let’s break it down:
Write the ground state electron configurations for the ions Mg²⁺ and Sr²⁺:
- Mg²⁺: 1s² 2s² 2p⁶
- Sr²⁺: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 4p⁶
Predict which ion has a larger ionic radius, Mg²⁺ or Sr²⁺:
- Sr²⁺ has a larger ionic radius because it has more occupied electron shells (4th energy level for Sr²⁺ vs. 2nd energy level for Mg²⁺).
Compare the lattice energy of MgCl₂ and SrCl₂:
- MgCl₂ has a higher lattice energy because Mg²⁺ is smaller than Sr²⁺, meaning the attraction between ions in MgCl₂ is stronger.
Final Takeaway:
Lattice energy and melting points are determined by the charges of the ions and the distance between them (based on their sizes). The smaller the ions and the higher the charges, the stronger the electrostatic forces and the higher the lattice energy.
By keeping Coulomb’s Law in mind, you can accurately predict lattice energy, melting points, and other properties of ionic solids!
Recent Comments


2.4 Structure of Metals and Alloys


Discover the Ultimate Multiplication Tables Generator – A Comprehensive Guide


Basic Algebra Concepts: Lesson 2.2


Basic Algebra Concepts: Lesson 1.4

