2.2 Intramolecular Force and Potential Energy


Table of Contents
ToggleIntramolecular Forces
You may be thinking, what exactly are intramolecular forces? These are the forces between atoms within a molecule! This is different from intermolecular forces, which we will cover in Unit 3.
💡 Intramolecular vs. Intermolecular Forces:
- Intermolecular forces act between molecules (think inter = between two groups).
- Intramolecular forces are within molecules, acting between atoms that make up a molecule (think intra = within a group).
So far, we’ve discussed two types of intramolecular forces:
- Covalent bonds
- Ionic bonds
👉 Need a quick review? Check out our study guide on the types of chemical bonds to help distinguish between ionic and covalent bonds.
Potential Energy and Bonding
You might wonder: where does potential energy fit into bonding? Remember, chemistry always strives for the most stable arrangement. The lower the potential energy in a bond, the more stable it is!
Because of this, we can describe physical and chemical processes using energy diagrams. A graph of potential energy vs. distance between atoms is a useful way to visualize bonding interactions. Here’s what you should know:
- Equilibrium bond length: The distance between two atoms where potential energy is lowest—i.e., where the atoms are most stable.
- Bond energy: The energy required to separate two atoms in a bond. It’s calculated as the difference in potential energy between separated atoms and atoms at their equilibrium bond length (most stable state).
- Bond strength: This correlates with bond energy. Higher bond energy means a stronger bond and more stability, while lower bond energy means a weaker, less stable bond.
- Bond length: The physical distance between two bonded atoms.
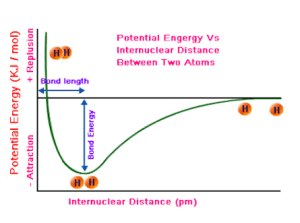
Potential Energy and Covalent Bonds
In molecular compounds with covalent bonds, the bond length is influenced by both atom size and bond order.
| Bond Order | Electrons Involved | Bond Length | Bond Energy |
|---|---|---|---|
| Single Bond (-) | Two electrons | Longest | Smallest |
| Double Bond (=) | Four electrons | Middle | Middle |
| Triple Bond (≡) | Six electrons | Shortest | Largest |
Remember: Each dash in a Lewis dot diagram represents two shared electrons.
• H—H single bond
• C═O double bond
• N≡N triple bond
Since bonds with higher bond energies are stronger and more stable, triple bonds are generally the most stable (and hardest to break). However, stability also depends on factors such as atomic size and charge.
Breaking Down a Potential Energy Diagram
For covalent bonds, bond length is influenced by the bond order (single, double, triple) and the balance between repulsive and attractive forces. The bond energy corresponds to the amount of energy required to break the bond.
Here’s a breakdown of each stage on the potential energy diagram:
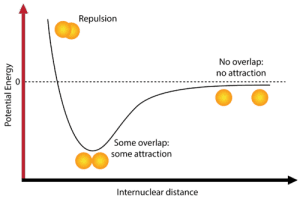

- Repulsion: When atoms are very close together, electron-electron repulsion causes instability, leading to potential energy > 0.
- Overlap/Attraction: At the equilibrium bond length, attractive and repulsive forces are balanced ⚖️, resulting in maximum stability (lowest potential energy).
- No overlap/Attraction: When atoms are far apart, there is no interaction, and potential energy approaches zero (no bond forms).
![Image Courtesy of SplainScience]
Example with Potential Energy Diagrams
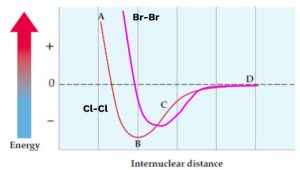
Consider a potential energy diagram for Cl-Cl. Where would Br-Br fall in comparison?
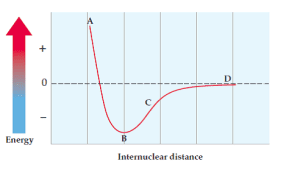

We use periodic trends to answer this:
- Internuclear distance: Br-Br will have a longer bond than Cl-Cl because bromine is larger (lower on the periodic table, meaning larger atomic radius).
- Potential energy: The Br-Br bond will be easier to break because bromine has a lower ionization energy than chlorine. This means the Br-Br bond will have less bond energy.
So, the Br-Br curve would be to the right (longer distance) and up (less energy) compared to the Cl-Cl curve.


Forces Within Ionic Bonds
Understanding the strength of ionic interactions relies on Coulomb’s Law.
You don’t need to memorize the formula for AP Chemistry, but you do need to understand its concepts:
- The energy between two charged particles (ions) depends on their charges and the distance between their nuclei.
- Stronger attraction occurs with greater charges and smaller distances between ions.
For example, small, highly charged ions (like Mg²⁺ and O²⁻) will have the strongest ionic interactions, leading to higher bond energies.
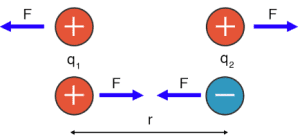

In this diagram, F represents force, q₁ and q₂ represent the charges of the ions, and r represents the distance between their nuclei.
Coulomb’s Law explains why opposites attract:
- Attraction occurs between oppositely charged ions (positive and negative).
- Repulsion occurs between ions with the same charge (both positive or both negative).
Recent Comments


2.4 Structure of Metals and Alloys


Discover the Ultimate Multiplication Tables Generator – A Comprehensive Guide


Basic Algebra Concepts: Lesson 2.2


Basic Algebra Concepts: Lesson 1.4

