1.8 Valence Electrons and Ionic Compounds


1.8 Valence Electrons and Ionic Compounds
In AP Chemistry, understanding valence electrons is crucial. Let’s do a quick review of what you should know so far:
- Valence electrons are the outermost electrons of an atom.
- These electrons are found in the s and p orbitals of the outermost shell.
- A gap in ionization energies helps identify how many valence electrons an element has.
- Elements in the same group on the periodic table have the same number of valence electrons.
Table of Contents
ToggleFoundational Knowledge of Bonding: Valence Electrons
The periodic table can tell us how many valence electrons an element has. These electrons determine how atoms bond with each other.
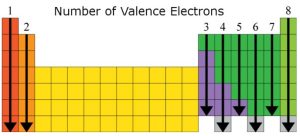

The image skips transition metals as they are less predictable and not the primary focus of the AP Chemistry exam. However, it’s beneficial to be familiar with some transition metals like Co, Cu, and Zn.
For example, you can see that oxygen has 6 valence electrons, and carbon has 4. The number of valence electrons affects how an element bonds. Elements in the same group often bond similarly and form compounds with similar properties. For example:
- Group 1 elements all bond with chlorine: LiCl, NaCl, KCl, RbCl.
- Group 2 elements all bond with oxygen: MgO, CaO, SrO, BaO.
Charges of Ions
Before we dive into chemical bonds, it’s essential to memorize the charges of most elements when they form compounds. This will help us understand what happens when elements bond.
Ions are charged atoms or molecules that have either gained or lost electrons. Atoms often form ions to achieve a more stable electron configuration. Keep in mind that stability is key in chemistry! ⚖️
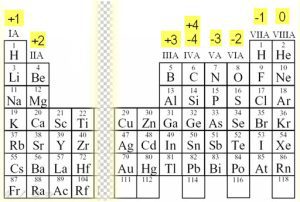
Again, the transition metals aren’t included because they have various charges and unique properties. Luckily, the AP exam won’t require you to memorize these. At most, you might need to write their electron configurations.
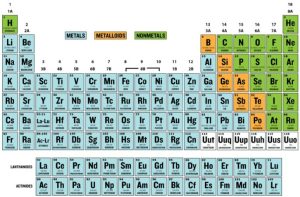
Types of Elements
There are three main types of elements:
- Metals, which are good conductors of heat and electricity, shiny, malleable (bendable), and ductile (can be drawn into a wire).
- Nonmetals, which are poor conductors of heat and electricity and are brittle.
- Metalloids, which exhibit properties of both metals and nonmetals. There are only seven metalloids.

Electronegativity
Electronegativity measures how strongly an atom attracts electrons from another atom. This concept is key when atoms share valence electrons, as it affects the pull on the electrons.
Electronegativity is one of the five major periodic trends you’ll need to understand for AP Chemistry. Remember that fluorine is the most electronegative element, with a value of 4.0. You can compare other elements’ electronegativities based on how close they are to fluorine on the periodic table.
👉 Need a quick review on electronegativity? Check out our study guide on Periodic Trends.
Types of Bonds
Atoms bond to lower their energy and increase their stability. There are two major types of bonds you’ll need to know for the AP Chemistry exam: ionic bonds and covalent bonds.
Ionic Bonds
Ionic bonds are formed through the transfer of electrons from one atom to another, usually from a metal to a nonmetal.
- The atom that loses electrons becomes positively charged and is called a cation (typically a metal).
- The atom that gains electrons becomes negatively charged and is called an anion (typically a nonmetal).
Properties of ionic compounds include strong bonds, solubility in water, and high conductivity of heat and electricity.
Example: NaCl (Sodium Chloride)
In NaCl, sodium (Na) loses one electron, becoming positively charged, while chlorine (Cl) gains an electron and becomes negatively charged.
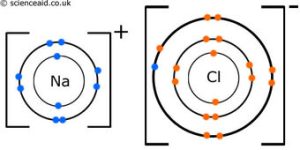

The valence electron in sodium is transferred to chlorine, allowing both ions to achieve a full octet. According to the octet rule, atoms are most stable when they have eight valence electrons.
Group 1 elements and Group 17 elements (halogens) often bond in this way. By forming ionic bonds, group 1 elements lose their outermost electron shell and achieve the electron configuration of the nearest noble gas.
When sodium and chlorine form ions, their electron configurations match those of neon and argon, respectively:
Na (Sodium):
1s² 2s² 2p⁶ 3s¹
Note: Sodium has one electron in its 3s orbital.
Na⁺ (Sodium Ion) / Ne (Neon):
1s² 2s² 2p⁶
Note: When sodium loses one electron to form Na⁺, it has the same electron configuration as neon (Ne).
Cl (Chlorine):
1s² 2s² 2p⁶ 3s² 3p⁵
Note: Chlorine has five electrons in its 3p orbital.
Cl⁻ (Chloride Ion) / Ar (Argon):
1s² 2s² 2p⁶ 3s² 3p⁶
Note: When chlorine gains one electron to form Cl⁻, it has the same electron configuration as argon (Ar).
- Na⁺ → [Ne]
- Cl⁻ → [Ar]
Covalent Bonds
Covalent bonds form when two atoms share electrons, typically between two nonmetals. Covalent bonds have low melting points and weak electrical conductivity.
There are two types of covalent bonds:
- Polar covalent bonds: Electrons are shared unequally between two atoms due to differing electronegativities.
- Nonpolar covalent bonds: Electrons are shared equally, often between atoms of the same nonmetal with similar electronegativities.
Covalent bonds are strong and stable, and they are responsible for the structure of many common substances like water (H₂O), methane (CH₄), carbon dioxide (CO₂), and even proteins, DNA, and carbohydrates! 🤯
Polar-Covalent Example: HF (Hydrogen Fluoride)
In hydrogen fluoride (HF), hydrogen and fluorine form a polar covalent bond. Fluorine, with an electronegativity of 4.0, strongly attracts electrons, resulting in an unequal distribution of electron density.
Think of it like this: fluorine is extremely “greedy” for electrons as it wants to fulfill its octet of eight valence electrons. As a result, it pulls the shared electron pair closer to itself.
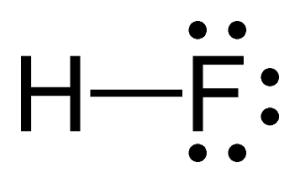
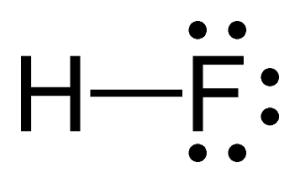
In this bond, note the difference from an ionic bond. Instead of a transfer of electrons, hydrogen and fluorine are sharing electrons. The dash between them represents the two shared electrons. Fluorine gets the shared electron from hydrogen, allowing both atoms to achieve a full outer shell.
Nonpolar-Covalent Example: Cl₂ (Chlorine Molecule)
In the covalent molecule Cl₂, two chlorine atoms share one electron each to complete their octet.
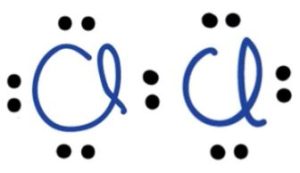

Just like in a polar covalent bond, electrons are shared. However, here both atoms are of the same element. This means they have identical electronegativities, so they exert equal pull on the electrons. Thus, the electrons are shared equally between the two atoms.
Not all nonpolar covalent bonds occur between identical atoms. Carbon-oxygen bonds in carbon dioxide (CO₂) and carbon-hydrogen bonds in methane (CH₄) are also nonpolar, despite different elements being involved. Here’s a comparison of their electronegativities:
- Electronegativity of hydrogen: 2.2
- Electronegativity of carbon: 2.55
- Electronegativity of oxygen: 3.44
Charges and Partial Charges in Bonds
When two atoms bond, the distribution of charge varies depending on their electronegativities.
- In nonpolar covalent bonds, where the electronegativities of the atoms are nearly the same, the electrons are shared equally. This results in a neutral charge distribution.
- In polar covalent bonds, a significant difference in electronegativities causes one atom to attract the electrons more strongly. The result is a partial charge on each atom:
- The atom with higher electronegativity (like fluorine) gains a partial negative charge (δ⁻).
- The atom with lower electronegativity (like hydrogen) gains a partial positive charge (δ⁺).
For example, in HF, fluorine’s higher electronegativity pulls the shared electrons closer to it, causing the fluorine to become partially negative and the hydrogen to become partially positive.
We use the Greek letter delta (δ) to represent partial charges. This symbol helps distinguish between partial charges in polar covalent bonds and full charges in ionic bonds.
Ionic Bonds and Full Charges
In an ionic bond, electrons are fully transferred from one atom to another, resulting in ions with complete opposite charges.
For instance, in the ionic compound NaCl:
- Sodium (Na) loses an electron, becoming Na⁺ with a +1 charge.
- Chlorine (Cl) gains an electron, becoming Cl⁻ with a -1 charge.
The reason metals tend to lose electrons is due to their low electronegativities. For example:
- Sodium (Na) has an electronegativity of 0.93, while chlorine (Cl) has an electronegativity of 3.16.
- This difference is much larger than in HF, which is why ionic bonds result in full charges rather than partial ones.
Key Concept: Electronegativity and Charge Distribution
- Low electronegativity atoms (like metals) are more likely to donate electrons and become cations (positive ions).
- High electronegativity atoms (like nonmetals) are more likely to accept electrons and become anions (negative ions).
Check Your Understanding: Quick Quiz
Here are some practice questions to test your understanding of ionic bonds:
- Atoms of calcium (Ca) combine with atoms of bromine (Br) to form an ionic bond.
- What ratio would they combine in?
- What other compounds have this same ratio with calcium?
- What elements could form an ionic bond with sulfur?
Answers
- Calcium and bromine bond in a 1:2 ratio, forming the compound CaBr₂. Calcium has a +2 charge, while bromine has a -1 charge. For a neutral compound, two bromine atoms are required for each calcium atom.
- Other compounds that have the same 1:2 ratio with calcium include compounds formed with any group 17 (halogens), like CaF₂ (calcium fluoride), CaCl₂ (calcium chloride), and CaI₂ (calcium iodide).
- Sulfur can bond with group 2 elements in a 1-to-1 ratio, such as MgS, CaS, and BaS. Alternatively, it can bond with group 1 elements in a 2-to-1 ratio, like Na₂S.
Recent Comments


1.8 Valence Electrons and Ionic Compounds


1.5 Atomic Structure and Electron Configuration


Discover the Ultimate Multiplication Tables Generator – A Comprehensive Guide


Basic Algebra Concepts: Lesson 2.2


Basic Algebra Concepts: Lesson 1.4

