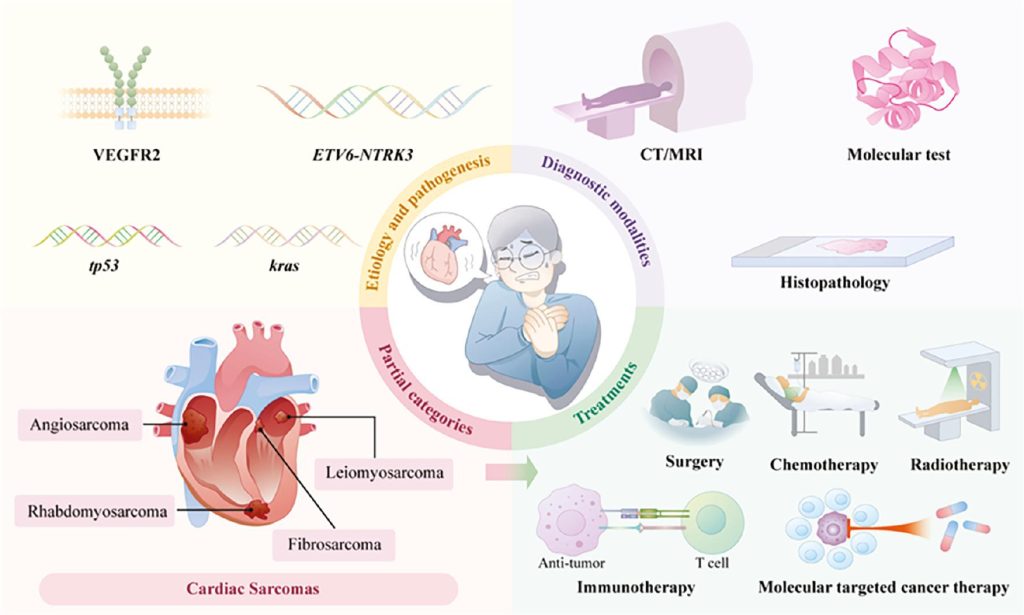
Sarcomas are a diverse group of cancers that arise from connective tissues—such as bone, muscle, fat, and cartilage—and account for roughly 1% of all adult cancers. Yet, these tumors are notoriously challenging due to their varied presentations and complex origins. In this post, we delve deep into the etiology of sarcoma, exploring the multiple factors—from genetics and environmental exposures to immune dysregulation and molecular pathways—that contribute to its development. Whether you’re a patient, caregiver, or simply interested in understanding more about sarcoma, this guide will provide you with a thorough and accessible overview.
Introduction
Imagine facing a diagnosis of a rare cancer that can emerge in the muscles, bones, or even the fat beneath your skin. Despite advances in oncology, sarcomas remain mysterious and challenging to treat because of their diverse origins and behaviors. Did you know that while sarcomas account for only about 1% of adult cancers, they represent up to 20% of childhood cancers? The etiology of sarcoma is not linked to a single cause—instead, it results from a complex interaction of genetic predispositions, environmental exposures, and molecular disruptions.
In this post, we will explore:
- What sarcoma is and how it differs from other cancers.
- The etiology of sarcoma, including key genetic and environmental factors.
- Historical milestones that have shaped our current understanding.
- In-depth exploration of the cellular and molecular mechanisms behind sarcoma development.
- Real-world examples and case studies that illustrate these concepts.
- Modern trends and emerging research in the field.
- Common misconceptions about sarcoma’s causes.
- Practical applications of this knowledge in improving patient care and guiding future research.
Understanding the etiology of sarcoma is crucial for early diagnosis, targeted therapies, and ultimately improving outcomes for patients. Let’s dive in and uncover what is known about the origins and driving forces behind sarcoma.
What Is Sarcoma?
Sarcomas are malignant tumors that develop from mesenchymal tissues—these include bones, muscles, fat, nerves, blood vessels, and connective tissues. There are two major categories:
- Bone Sarcomas: Such as osteosarcoma and Ewing sarcoma.
- Soft Tissue Sarcomas: Encompassing a wide variety of subtypes like liposarcoma, leiomyosarcoma, and synovial sarcoma.
Key Characteristics of Sarcomas:
- Diverse Origins: Unlike carcinomas (which arise from epithelial cells), sarcomas originate from the body’s supportive or connective tissues.
- Heterogeneous Behavior: Sarcomas vary widely in aggressiveness, treatment response, and prognosis.
- Rarity in Adults, Prevalence in Children: They are relatively rare among adults but represent a significant proportion of pediatric malignancies.
- Diagnosis and Treatment Challenges: Their varied presentations and rarity often delay diagnosis and complicate treatment strategies.
For more background, the Wikipedia article on Sarcoma provides an excellent primer on the topic.
Defining the Etiology of Sarcoma
The etiology of sarcoma refers to the study of its underlying causes and contributing factors. Sarcomas do not typically result from a single cause; rather, their development is influenced by a constellation of factors that can be broadly grouped into:
- Genetic Factors: Inherited mutations or spontaneous genetic alterations that predispose individuals to sarcoma.
- Environmental Exposures: External agents such as radiation, chemicals, or viral infections that trigger malignant transformation.
- Immune Dysregulation: Abnormalities in the immune system that may fail to recognize and eliminate emerging cancer cells.
- Molecular and Cellular Mechanisms: Disruptions in cellular signaling, apoptosis, and repair mechanisms that promote uncontrolled cell growth.
- Epigenetic Changes: Modifications in gene expression that do not involve changes in the DNA sequence but can affect cancer risk.
By examining these factors, researchers can piece together how sarcomas form and progress, paving the way for improved diagnostics and targeted therapies.
Historical and Contextual Background
Early Observations and Milestones
The history of sarcoma research spans well over a century:
- Early Descriptions: The term “sarcoma” was first introduced in the 19th century to describe malignant tumors of connective tissue. Early clinicians noted the distinct behavior of these tumors compared to carcinomas.
- Classification and Subtyping: Over time, advances in histopathology led to the classification of sarcomas into bone and soft tissue types. Landmark discoveries in cytogenetics revealed specific chromosomal translocations (such as the t(11;22) translocation in Ewing sarcoma) that helped define subtypes.
- Genetic Insights: The latter half of the 20th century saw significant progress in understanding the genetic basis of sarcomas. Identification of gene fusions and mutations provided critical insights into the molecular mechanisms driving these tumors.
Shifting Paradigms
- From Environmental to Genetic Causes: Initially, environmental exposures—such as radiation—were considered the primary cause of sarcomas. However, the discovery of specific genetic mutations and hereditary cancer syndromes (e.g., Li-Fraumeni syndrome, which involves p53 mutations) has shifted the focus to a multifactorial etiology.
- Emergence of Molecular Pathways: Advances in molecular biology have revealed that sarcomas are not a single disease but a collection of disorders with distinct molecular signatures. This has led to the development of targeted therapies aimed at specific pathways (e.g., tyrosine kinase inhibitors in gastrointestinal stromal tumors).
Notable Historical Anecdotes
- Radiation-Induced Sarcomas: One of the first clues to the complex etiology of sarcoma came from observations of patients who developed sarcomas years after receiving radiation therapy for other cancers. This provided early evidence of environmental triggers interacting with individual susceptibility.
- Hereditary Syndromes: The identification of familial cancer syndromes, such as Li-Fraumeni syndrome, underscored the role of inherited genetic mutations in predisposing individuals to sarcomas, among other cancers.
These historical developments set the stage for today’s comprehensive understanding of the etiology of sarcoma.
In-Depth Exploration of the Etiology of Sarcoma
To fully grasp the etiology of sarcoma, we must explore its multiple contributing factors in detail.
5.1 Genetic Factors
Inherited and Somatic Mutations
Genetic alterations are central to the development of sarcomas:
- Hereditary Predisposition: In some families, inherited mutations significantly increase the risk of sarcoma. For example, Li-Fraumeni syndrome, caused by mutations in the TP53 gene, predisposes individuals to various sarcomas and other cancers.
- Somatic Mutations: Most sarcomas result from acquired (somatic) mutations that occur in individual cells. These mutations can be spontaneous or triggered by environmental exposures, such as radiation or chemical carcinogens.
Specific Genetic Aberrations
- Chromosomal Translocations: Many soft tissue sarcomas are characterized by specific chromosomal translocations that create fusion genes. For example, the EWS-FLI1 fusion gene in Ewing sarcoma is a well-known driver mutation.
- Gene Amplifications and Deletions: Abnormalities such as gene amplifications (e.g., MDM2 amplification in liposarcoma) or deletions can disrupt normal cell cycle control and promote malignant transformation.
- Mutations in Signaling Pathways: Mutations affecting key cellular signaling pathways, such as those involving receptor tyrosine kinases or the PI3K/AKT/mTOR pathway, contribute to uncontrolled cell growth and survival.
Example:
A young patient diagnosed with Ewing sarcoma may harbor a translocation between chromosomes 11 and 22, resulting in the EWS-FLI1 fusion gene—a molecular hallmark that drives the malignant behavior of the tumor.
5.2 Environmental Exposures
Radiation Exposure
- Radiation-Induced Sarcoma: Exposure to ionizing radiation is a well-documented environmental risk factor for sarcoma. Patients who have undergone radiation therapy for other cancers sometimes develop sarcomas years later in the irradiated field.
- Mechanism: Radiation can cause direct DNA damage, leading to mutations, chromosomal rearrangements, and genomic instability, which in turn can trigger the malignant transformation of cells.
Chemical Carcinogens
- Occupational Exposures: Certain chemicals and industrial pollutants have been linked to an increased risk of sarcoma. For example, exposure to herbicides, pesticides, and industrial chemicals (such as vinyl chloride) has been implicated in some cases.
- Lifestyle Factors: In addition to occupational hazards, lifestyle-related exposures—such as those encountered by individuals working in certain industries—can contribute to sarcoma risk.
Viral Infections
- Oncoviruses: Certain viruses have been proposed to play a role in the etiology of sarcoma. For instance, the Kaposi sarcoma–associated herpesvirus (KSHV) is known to cause Kaposi sarcoma, particularly in immunocompromised individuals.
- Mechanism: Oncoviruses may integrate into the host genome, disrupt normal gene regulation, or produce viral proteins that interfere with cell cycle control and promote oncogenesis.
5.3 Immune Dysregulation and Inflammatory Pathways
Chronic Inflammation
- Role in Carcinogenesis: Chronic inflammation is a recognized risk factor for many cancers, including sarcomas. Inflammatory cells release reactive oxygen and nitrogen species, cytokines, and growth factors that can damage DNA and promote cell proliferation.
- Inflammatory Microenvironment: In some cases, an underlying inflammatory condition or autoimmune disorder may predispose individuals to sarcoma development by creating a pro-tumorigenic microenvironment.
Immune Surveillance Failure
- Immune Evasion: Normally, the immune system detects and eliminates abnormal cells through immune surveillance. However, defects in immune function can allow mutated cells to escape detection and progress to cancer.
- Immunosuppressive Therapies: Patients receiving long-term immunosuppressive medications (for autoimmune diseases or organ transplantation) have an increased risk of developing sarcomas, likely due to reduced immune surveillance.
5.4 Oxidative Stress and DNA Damage
Generation of Reactive Oxygen Species (ROS)
- Mechanism: Environmental exposures like radiation and certain chemicals, as well as chronic inflammation, generate ROS. These reactive molecules can cause direct damage to DNA, proteins, and lipids.
- DNA Damage: Accumulated oxidative DNA damage can lead to mutations, strand breaks, and chromosomal instability—all of which contribute to carcinogenesis.
Antioxidant Defense Systems
- Imbalance: When the balance between ROS production and antioxidant defenses is disrupted, cells become more susceptible to genetic damage. This imbalance is a key factor in the development of sarcoma, as well as many other cancers.
5.5 Structural and Microenvironmental Factors
Tissue Microenvironment
- Extracellular Matrix (ECM): The ECM provides structural support to tissues and regulates cellular behavior. Alterations in ECM composition or degradation by proteolytic enzymes can facilitate tumor invasion and metastasis.
- Stromal Cells: Fibroblasts, immune cells, and vascular cells in the tumor microenvironment interact with cancer cells, often promoting their growth and survival.
Angiogenesis
- New Blood Vessel Formation: Tumors, including sarcomas, stimulate angiogenesis to secure a blood supply that delivers oxygen and nutrients necessary for growth. Factors such as vascular endothelial growth factor (VEGF) play a central role.
- Therapeutic Target: Anti-angiogenic therapies have emerged as potential treatments for sarcomas by disrupting the tumor’s blood supply.
5.6 Emerging Concepts: Epigenetics and the Tumor Microenvironment
Epigenetic Modifications
- DNA Methylation: Changes in DNA methylation patterns can lead to the silencing of tumor suppressor genes or the activation of oncogenes without altering the DNA sequence.
- Histone Modifications: Alterations in histone acetylation or methylation can affect chromatin structure and gene expression, contributing to the malignant phenotype.
- MicroRNAs: Dysregulated microRNA expression can influence the expression of genes involved in cell proliferation, apoptosis, and metastasis.
- Reversibility: One of the promising aspects of epigenetic modifications is their reversible nature, which opens up new avenues for targeted therapies.
The Tumor Microenvironment
- Crosstalk Between Cells: The dynamic interaction between cancer cells and their surrounding stroma (including immune cells, fibroblasts, and endothelial cells) is crucial in tumor development.
- Immune Modulation: Some sarcomas actively modulate the immune response to escape detection. Understanding these interactions is key to developing immunotherapies.
Real-World Example:
A patient diagnosed with a soft tissue sarcoma may undergo genetic testing that reveals not only a specific chromosomal translocation but also abnormal methylation patterns in key tumor suppressor genes. This information can guide personalized treatment strategies, such as the use of targeted therapies that inhibit specific molecular pathways or epigenetic drugs aimed at reversing aberrant gene silencing.
Real-World Examples and Case Studies
Case Study 1: Genetic Predisposition and Hereditary Syndromes
Consider Emily, a 30-year-old woman from a family with a history of Li-Fraumeni syndrome. Genetic screening reveals a mutation in the TP53 gene, which significantly increases her risk of developing various cancers, including sarcomas. Despite leading a healthy lifestyle, Emily is diagnosed with a rare soft tissue sarcoma. Her case underscores the role of inherited genetic mutations in the etiology of sarcoma and highlights the importance of genetic counseling and early screening in high-risk populations.
Case Study 2: Radiation-Induced Sarcoma
John, a 55-year-old man, underwent radiation therapy for Hodgkin lymphoma 20 years ago. Recently, he developed a new tumor in the irradiated field that was diagnosed as a radiation-induced sarcoma. This case exemplifies how environmental exposure to ionizing radiation can lead to secondary malignancies years after the initial treatment, providing a clear illustration of the environmental component in the etiology of sarcoma.
Case Study 3: Chemical Exposure and Occupational Risk
Carlos, a 48-year-old construction worker, was frequently exposed to industrial chemicals and asbestos during his career. Although he never smoked, he developed an aggressive form of bone sarcoma. His diagnosis highlights how chronic exposure to chemical carcinogens in certain occupations can trigger the development of sarcomas, even in the absence of traditional risk factors like smoking.
Case Study 4: Epigenetic Alterations and Microenvironmental Influences
A recent research study followed a cohort of sarcoma patients and analyzed their tumor samples for epigenetic changes. One patient, Mark, showed distinct patterns of DNA hypomethylation in regions regulating cell cycle control and apoptosis. In addition, his tumor microenvironment was rich in inflammatory cells and pro-angiogenic factors, suggesting that both epigenetic modifications and microenvironmental factors contributed to his tumor’s aggressive behavior. This case illustrates the emerging concept that not only genetic mutations but also reversible epigenetic changes play a pivotal role in the etiology of sarcoma.
Importance and Applications
Clinical Implications
Understanding the etiology of sarcoma is essential for several reasons:
- Early Diagnosis: Identifying genetic markers, environmental risk factors, and epigenetic signatures can help detect sarcomas at an earlier stage, potentially improving outcomes.
- Targeted Therapies: A deep knowledge of the molecular pathways involved in sarcoma development has led to the development of targeted therapies. For example, tyrosine kinase inhibitors are now used in the treatment of gastrointestinal stromal tumors (GISTs), a type of sarcoma.
- Personalized Medicine: Genetic and molecular profiling of sarcomas allows oncologists to tailor treatment plans to the individual patient’s tumor characteristics, optimizing efficacy while minimizing side effects.
- Preventive Strategies: For individuals with known hereditary predispositions, such as Li-Fraumeni syndrome, proactive surveillance and lifestyle modifications may reduce risk or enable early intervention.
Societal and Economic Impact
- Healthcare Costs: Sarcomas often require complex and expensive treatments, including surgery, radiation, and targeted therapies. Early detection and personalized treatment strategies can reduce the overall financial burden on healthcare systems.
- Quality of Life: More effective treatments not only extend survival but also improve the quality of life for patients by reducing symptoms and treatment-related complications.
- Public Health Policies: Understanding environmental risk factors—such as occupational exposures and radiation—can inform regulations and workplace safety standards, ultimately reducing the incidence of sarcoma in high-risk populations.
Research and Innovation
- Biomarker Development: Ongoing research is focused on identifying reliable biomarkers for sarcoma, which can aid in diagnosis, monitor treatment response, and predict outcomes.
- Novel Therapeutics: Advances in molecular biology have led to promising new treatments, including immunotherapies and epigenetic drugs, which target the specific mechanisms underlying sarcoma.
- Interdisciplinary Collaboration: The complex nature of sarcoma’s etiology necessitates collaboration among geneticists, oncologists, immunologists, and environmental scientists, fostering innovation and accelerating the pace of discovery.
Common Misconceptions and FAQs
Common Misconceptions
Misconception 1: Sarcomas are caused solely by genetic mutations.
Reality: While genetic mutations and hereditary syndromes are important, the etiology of sarcoma is multifactorial. Environmental exposures (such as radiation and chemicals), immune dysregulation, and epigenetic modifications all play critical roles.Misconception 2: All sarcomas are the same.
Reality: Sarcomas are a highly heterogeneous group of cancers with diverse origins, behaviors, and responses to treatment. Bone sarcomas differ markedly from soft tissue sarcomas, and even within these categories, subtypes have distinct etiologies and molecular profiles.Misconception 3: Sarcoma only affects children and young adults.
Reality: Although some sarcomas (like Ewing sarcoma) are more common in younger populations, many types of sarcoma, including certain bone and soft tissue sarcomas, are diagnosed in adults.Misconception 4: Environmental exposures are irrelevant if you don’t have a family history of cancer.
Reality: Even in the absence of a strong genetic predisposition, environmental factors—such as radiation and chemical exposures—can independently trigger the development of sarcoma.
Frequently Asked Questions (FAQs)
Q1: What does “etiology of sarcoma” mean?
A: It refers to the study of the causes and contributing factors that lead to the development of sarcoma. These factors include genetic mutations, environmental exposures, immune system dysregulation, epigenetic changes, and alterations in the tumor microenvironment.
Q2: How significant are genetic factors in sarcoma?
A: Genetic factors play a crucial role, particularly in hereditary syndromes like Li-Fraumeni syndrome, where mutations in the TP53 gene significantly increase sarcoma risk. However, most sarcomas also involve somatic mutations acquired during a person’s lifetime.
Q3: Can lifestyle changes reduce the risk of developing sarcoma?
A: While many sarcomas occur sporadically, minimizing exposure to known environmental carcinogens (such as ionizing radiation and certain industrial chemicals) and adhering to a healthy lifestyle may help lower overall cancer risk.
Q4: What role does radiation play in the etiology of sarcoma?
A: Radiation exposure is a well-established risk factor for sarcoma. Patients who receive radiation therapy for other cancers may develop radiation-induced sarcomas years later due to DNA damage and chromosomal alterations.
Q5: Are there new treatments targeting the molecular causes of sarcoma?
A: Yes. Advances in molecular biology have led to targeted therapies, such as tyrosine kinase inhibitors for GIST, as well as emerging immunotherapies and epigenetic drugs designed to target specific genetic and molecular pathways involved in sarcoma development.
Q6: What are the most common types of sarcoma?
A: Sarcomas are broadly divided into bone sarcomas (e.g., osteosarcoma, Ewing sarcoma) and soft tissue sarcomas (e.g., liposarcoma, leiomyosarcoma, synovial sarcoma). Each type has its own unique etiology and clinical characteristics.
Modern Relevance and Current Trends
Advances in Genetic and Molecular Research
- High-Throughput Genomics: Modern sequencing technologies have identified numerous genetic mutations and chromosomal translocations that drive various sarcoma subtypes. These discoveries are paving the way for personalized therapies.
- Targeted Therapy: Drugs such as imatinib for GIST, which target specific tyrosine kinases, exemplify how understanding the molecular basis of sarcoma can directly influence treatment.
- Gene Editing: Emerging technologies like CRISPR offer the potential to correct genetic defects associated with hereditary sarcoma syndromes.
Epigenetics and the Tumor Microenvironment
- Epigenetic Therapies: As researchers uncover the role of DNA methylation and histone modifications in sarcoma, new drugs targeting these epigenetic changes are being developed.
- Microenvironmental Research: Studies are increasingly focusing on how the tumor microenvironment—including stromal cells, immune cells, and the extracellular matrix—contributes to sarcoma growth and metastasis. These insights are leading to innovative treatment strategies that target not only the tumor cells but also their supportive environment.
Environmental and Occupational Health Initiatives
- Regulatory Measures: Public health initiatives aimed at reducing exposure to industrial carcinogens, improving workplace safety, and limiting environmental pollution are crucial in preventing radiation- and chemical-induced sarcomas.
- Awareness Campaigns: Increased public awareness about the risks associated with certain occupations and exposures can help mitigate the incidence of sarcoma in high-risk populations.
Integration of Multi-Omics Data
- Systems Biology Approaches: Researchers are now integrating genomic, epigenomic, proteomic, and metabolomic data to develop a holistic understanding of the etiology of sarcoma. This integrated approach promises to identify novel biomarkers and therapeutic targets.
- Personalized Medicine: With more precise molecular profiling, clinicians can tailor treatment regimens based on the unique genetic and molecular signature of each patient’s tumor, leading to improved outcomes.
Emerging Therapeutic Strategies
- Immunotherapy: Advances in immunotherapy, including checkpoint inhibitors and adoptive cell therapies, are showing promise in treating certain sarcoma subtypes by harnessing the body’s immune system.
- Combination Therapies: Ongoing clinical trials are exploring the efficacy of combining targeted therapies with conventional chemotherapy, radiation, and immunotherapy to overcome resistance and improve survival rates.
Conclusion and Call to Action
The etiology of sarcoma is a complex and multifaceted subject, encompassing genetic predispositions, environmental exposures, immune dysregulation, molecular alterations, and epigenetic modifications. This comprehensive exploration highlights that sarcomas are not caused by a single factor but rather by an intricate interplay of multiple elements that collectively drive the malignant transformation of connective tissues.
Key Takeaways:
- Multifactorial Origins: Sarcomas arise from a combination of inherited mutations, somatic genetic changes, environmental exposures (such as radiation and chemicals), and disturbances in the tumor microenvironment.
- Critical Role of Inflammation and Oxidative Stress: Chronic inflammation, oxidative stress, and a protease-antiprotease imbalance are central to the tissue destruction seen in many sarcomas.
- Modern Research Drives Innovation: Advances in genomics, epigenetics, and molecular biology have led to targeted therapies that offer hope for improved treatment outcomes.
- Prevention and Early Detection: Awareness of risk factors and early molecular and genetic screening can play a pivotal role in preventing and managing sarcoma.
- Interdisciplinary Collaboration: Understanding the etiology of sarcoma requires an integrated approach across multiple scientific disciplines, leading to innovations that can transform patient care.
Call to Action
If you found this guide on the etiology of sarcoma informative and helpful, please share it with your network—whether you’re a healthcare professional, a researcher, or someone personally affected by sarcoma. Stay informed by exploring reputable resources such as the National Cancer Institute, American Cancer Society, and the Sarcoma Foundation of America. We invite you to leave comments, ask questions, or share your own experiences below. Your feedback and engagement are invaluable in spreading awareness and advancing research in this challenging field.
Final Thoughts
The journey into the etiology of sarcoma reveals a landscape of complexity where genetics, environment, and molecular biology converge to shape disease development. With every new discovery, from chromosomal translocations to epigenetic modifications, our understanding deepens—offering promising avenues for early detection, personalized therapy, and ultimately, improved patient outcomes.
By staying informed and supporting ongoing research, we can work together to unravel the mysteries of sarcoma and pave the way toward a future where this formidable disease can be managed more effectively, or even prevented. Remember: knowledge is power, and together we can drive the innovation that transforms lives.