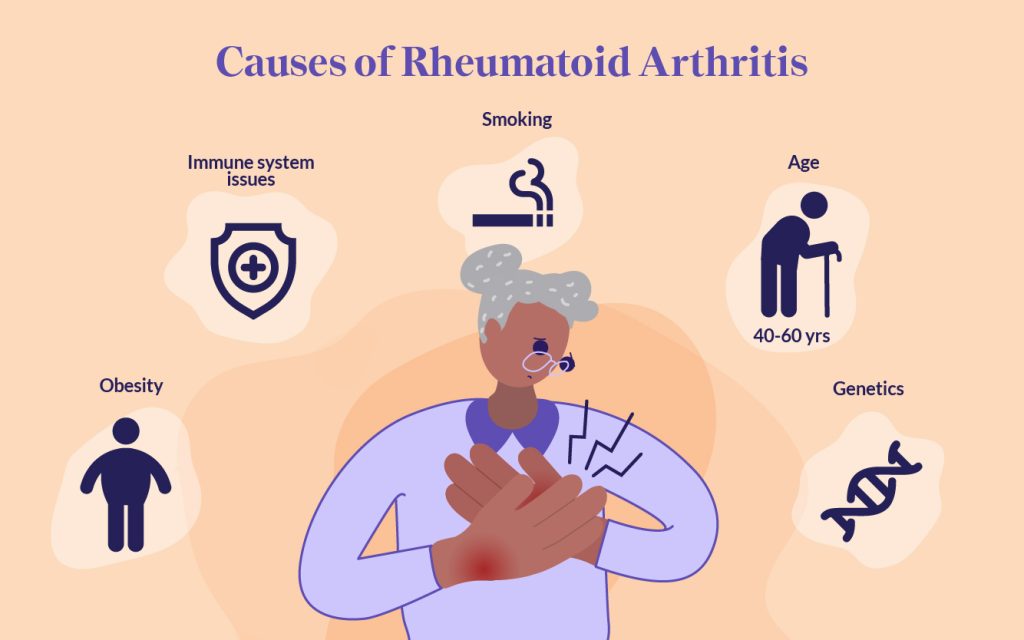
Rheumatoid arthritis (RA) is more than just joint pain—it’s a complex autoimmune disease that affects millions of people worldwide. Despite decades of research, the precise cause of RA remains elusive. However, scientists now understand that the etiology of rheumatoid arthritis is multifactorial, involving a mix of genetic predispositions, environmental triggers, immune system misfires, and even epigenetic changes. In this comprehensive guide, we’ll explore these contributing factors, trace the historical milestones that shaped our understanding of RA, and discuss modern trends in research and treatment. Whether you’re a patient, caregiver, or simply curious about this debilitating condition, this post is designed to empower you with knowledge.
Introduction
Imagine waking up every morning to stiff, painful joints that disrupt your daily life. For millions of people living with rheumatoid arthritis, this isn’t just an occasional inconvenience—it’s a relentless battle that affects every aspect of their well‐being. Although RA was once thought to be a simple “wear and tear” problem, research has revealed that its roots lie in a tangled web of genetic, environmental, immunologic, and epigenetic factors.
Did you know? Approximately 1% of adults worldwide suffer from RA, and women are two to three times more likely to develop the disease than men. Yet, despite its prevalence, the exact causes remain partly mysterious. In this post, we will delve deep into the etiology of rheumatoid arthritis, exploring:
- How genetics and family history contribute to RA risk.
- The environmental triggers—including smoking, pollutants, and infections—that can spark the disease.
- The immune system’s role in mistakenly attacking the body’s own tissues.
- The emerging influence of epigenetics and the microbiome on RA development.
- Historical breakthroughs that have reshaped our understanding.
- Practical insights and common misconceptions to help you navigate this complex condition.
By the end of this guide, you’ll have a clear understanding of what causes RA and why a multifaceted approach is essential for effective treatment and prevention.
What Is Rheumatoid Arthritis?
Rheumatoid arthritis is a chronic inflammatory disorder classified as an autoimmune disease. Unlike osteoarthritis—a condition caused by wear and tear—RA occurs when the immune system erroneously attacks the synovium, the thin layer of tissue lining the joints. This misguided immune response leads to persistent inflammation that can eventually damage the cartilage, bone, and surrounding tissues.
Key characteristics of RA include:
- Symmetrical Joint Involvement: RA typically affects the same joints on both sides of the body, such as both hands or both feet.
- Chronic Inflammation: Ongoing joint inflammation leads to pain, swelling, and stiffness, particularly in the mornings or after periods of inactivity.
- Systemic Impact: RA is a systemic disease that may also affect other organs, including the heart, lungs, eyes, and skin.
- Autoantibody Production: The immune system produces specific autoantibodies—such as rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) antibodies—that are hallmarks of the disease.
Understanding the etiology of rheumatoid arthritis means delving into the factors that set off this destructive immune response.
Defining the Etiology of Rheumatoid Arthritis
The etiology of rheumatoid arthritis refers to the study of its origins and underlying causes. In RA, no single factor is responsible; rather, the disease arises from a complex interplay of several components:
- Genetic Factors: Inherited genes significantly increase susceptibility.
- Environmental Triggers: Lifestyle factors, such as smoking and exposure to pollutants, can precipitate the disease.
- Immune System Dysregulation: A malfunctioning immune system mistakenly targets the body’s own tissues.
- Epigenetic Changes: Modifications in gene expression—not changes in the DNA sequence itself—may also drive RA.
- Microbiome Imbalances: Emerging research suggests that alterations in gut bacteria could influence immune responses and contribute to RA.
By understanding these elements, researchers hope to develop targeted therapies that address the root causes, leading to better management and possibly prevention of RA.
Historical Perspectives: How Our Understanding Evolved
Early Theories and Initial Discoveries
Historically, rheumatoid arthritis was often misunderstood. Early on, many believed that RA was simply a form of “wear and tear” or that it was caused by poor lifestyle choices. It wasn’t until the mid-20th century that scientists began to recognize RA as an autoimmune disorder.
- Rheumatoid Factor (RF) Discovery: In the 1940s and 1950s, researchers identified the presence of rheumatoid factor—a type of autoantibody—in patients with RA. This discovery shifted the focus from degenerative joint disease to an immune-mediated process.
- Identification of Anti-CCP Antibodies: Later, in the 1990s, the development of tests for anti-cyclic citrullinated peptide (anti-CCP) antibodies provided a more specific marker for RA. These antibodies helped to solidify the concept that RA is primarily driven by an autoimmune response.
Milestones in Genetic Research
- Shared Epitope Hypothesis: In the 1980s, researchers such as Peter Gregersen and colleagues proposed the “shared epitope” hypothesis. They discovered that certain variations in the HLA-DRB1 gene (a part of the major histocompatibility complex) were strongly associated with RA. This was a groundbreaking moment that linked genetic factors to the disease.
- Twin Studies and Family History: Subsequent twin and family studies further confirmed that genetics play a critical role in RA susceptibility, although environmental factors also have a significant impact.
Advances in Understanding Autoimmunity
- Citrullination and Autoantibody Production: Research in the 1990s and 2000s uncovered the role of protein citrullination—a process by which the amino acid arginine is converted into citrulline—in triggering an immune response. This finding helped explain the presence of anti-CCP antibodies in RA patients.
- Role of Inflammation: Scientists began to understand how chronic inflammation driven by cytokines such as tumor necrosis factor (TNF), interleukin-6 (IL-6), and interleukin-1 (IL-1) contributes to joint damage and systemic complications.
Modern Research and Emerging Trends
Today, our understanding of the etiology of rheumatoid arthritis continues to evolve. New studies are exploring:
- The impact of epigenetic changes, including DNA methylation and histone modifications.
- The role of the gut microbiome in modulating immune responses.
- Novel environmental triggers such as air pollution and occupational exposures.
- How gene-environment interactions set off the cascade of autoimmune responses.
Reputable sources such as the World Health Organization and Mayo Clinic offer up-to-date fact sheets that continue to inform both clinicians and patients alike.
In-Depth Exploration of the Etiology
The etiology of rheumatoid arthritis is multifaceted. Below, we break down the primary contributors into distinct categories.
Genetic Factors
The Role of Inherited Genes
Genetics play a substantial role in determining who is at risk for RA. Research shows that:
- Family History: Individuals with a close relative who has RA are at a higher risk. Twin studies suggest that the heritability of seropositive RA may be between 40% and 65%.
- HLA-DRB1 and the Shared Epitope: The strongest genetic association is found in the HLA-DRB1 gene. Specific alleles (such as HLA-DRB104, HLA-DRB101, and HLA-DRB1*10) contain a conserved sequence known as the “shared epitope.” This shared epitope is associated with an increased risk of developing ACPA-positive RA.
- Other Genes: Genome-wide association studies (GWAS) have identified additional genes linked to RA, including PTPN22, CTLA4, IL-2RA, STAT4, and TRAF1. Although each of these genes contributes only a small amount to overall risk, together they help shape an individual’s susceptibility.
Real-World Example:
Imagine two siblings who both inherit the same high-risk HLA-DRB1 allele. One sibling, who also smokes heavily and has a history of periodontal disease, develops RA in their 40s, while the other, who lives a healthier lifestyle, remains symptom-free. This scenario illustrates how genetic predisposition works in tandem with environmental factors.
Family and Twin Studies
- Twin Concordance Rates: Studies have shown that identical twins have a higher concordance rate for RA than fraternal twins. This supports the notion that genetics is a key contributor, though the fact that the rate is not 100% also highlights the role of environmental factors.
- Gene-Environment Interactions: Even with genetic susceptibility, the onset of RA often requires an environmental trigger, which leads us to the next major component of etiology.
Environmental Influences
Smoking and Air Pollution
Environmental factors are crucial in triggering RA in genetically susceptible individuals:
- Cigarette Smoking: One of the most consistently identified environmental risk factors is cigarette smoking. Smokers with a genetic predisposition (such as the shared epitope) have a dramatically increased risk of developing RA. Smoking is thought to promote protein citrullination in the lungs, leading to the formation of autoantibodies.
- Air Pollution: Exposure to pollutants—such as silica dust, asbestos, and chemicals from traffic emissions—has also been linked to an increased risk of RA. These pollutants may trigger inflammation and autoimmunity through oxidative stress and other mechanisms.
Infections and Periodontal Disease
- Infections: Although no specific infectious agent has been definitively linked to RA, researchers have investigated whether certain viruses or bacteria might trigger the autoimmune response. For example, some studies suggest that periodontal bacteria (e.g., Porphyromonas gingivalis) might contribute to protein citrullination and subsequent autoantibody production.
- Periodontal Disease: Poor dental hygiene and chronic gum infections have been associated with RA. The inflammation in the gums may release enzymes that modify proteins, making them targets for the immune system.
Diet, Obesity, and Lifestyle
- Dietary Factors: Emerging evidence suggests that diet plays a role in RA. A “western” diet—high in processed foods and low in fiber—may increase the risk, while diets rich in omega-3 fatty acids (found in fish) might be protective.
- Obesity: Excess weight is another modifiable risk factor. Obesity is linked to chronic low-grade inflammation, which can contribute to the development and severity of RA.
- Stress: Both physical and emotional stress have been implicated as triggers that can exacerbate autoimmune responses in susceptible individuals.
Quick Bullet Points on Environmental Factors:
- Smoking: Increases protein citrullination and risk in genetically predisposed individuals.
- Pollutants: Exposure to silica, asbestos, and vehicle emissions may trigger inflammation.
- Infections: Periodontal bacteria and other pathogens might initiate immune responses.
- Diet and Obesity: Unhealthy diets and excess body weight promote systemic inflammation.
- Stress: Both physical and emotional stress can exacerbate autoimmunity.
Autoimmunity and Immunologic Mechanisms
The Autoimmune Nature of RA
At its core, rheumatoid arthritis is an autoimmune disease—one in which the immune system mistakenly attacks the body’s own tissues. In RA:
- Immune Cells Go Awry: T cells, B cells, and macrophages are activated inappropriately. They infiltrate the synovial membrane (the lining of the joints) and trigger a chronic inflammatory response.
- Production of Autoantibodies: Two key autoantibodies in RA are rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA).
- Rheumatoid Factor (RF): An antibody that targets the Fc portion of immunoglobulin G (IgG). While RF is found in about 80% of RA patients, it can also be present in other diseases and even in some healthy individuals.
- Anti-Citrullinated Protein Antibodies (ACPA): These antibodies are highly specific for RA and are produced when the body’s proteins undergo a process called citrullination (the conversion of arginine into citrulline by the enzyme peptidylarginine deiminase, or PAD).
The Inflammatory Cascade in RA
Once the immune system is triggered:
- Cytokine Storm: Pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) are released. These molecules amplify the inflammatory response, leading to joint pain, swelling, and eventual destruction of cartilage and bone.
- Synovial Hyperplasia: The synovial membrane thickens due to the proliferation of fibroblast-like synoviocytes (FLS) and infiltration of immune cells. Over time, this can form a pannus—a tissue mass that invades and destroys adjacent bone and cartilage.
Real-World Scenario:
A patient with a genetic predisposition begins to experience joint pain and stiffness. Over time, autoantibodies such as ACPA become detectable in their blood years before clinical symptoms appear. As the immune system ramps up its inflammatory response, cytokine levels rise, leading to visible swelling and pain. Early intervention with medications that block TNF-α or IL-6 can help dampen this cascade and slow joint damage.
The Role of Cytokines
Key cytokines in the pathogenesis of RA include:
- TNF-α: Drives inflammation and joint destruction; targeted by many biologic DMARDs.
- IL-6: Promotes the inflammatory process and autoantibody production.
- IL-1: Contributes to cartilage breakdown and joint pain.
These cytokines not only cause local joint damage but also contribute to systemic manifestations such as fatigue, anemia, and an increased risk of cardiovascular disease.
Epigenetic Modifications
DNA Methylation
Epigenetics refers to heritable changes in gene expression that do not involve alterations to the DNA sequence. In rheumatoid arthritis:
- Hypomethylation: Many genes involved in the immune response are hypomethylated (i.e., have fewer methyl groups attached), leading to their overexpression. This can result in an exaggerated inflammatory response.
- Promoter Regions: Changes in DNA methylation in promoter regions of key genes may cause them to be “turned on” inappropriately in RA patients.
Histone Modifications
Histone proteins help package DNA in the nucleus, and their modification can alter gene expression:
- Acetylation: Histone acetylation generally increases gene expression. In RA, abnormal histone acetylation patterns may contribute to the overexpression of inflammatory genes.
- Deacetylation: Histone deacetylases (HDACs) normally remove acetyl groups. In RA, altered HDAC activity may further disrupt the balance of gene expression.
Non-Coding RNAs
- MicroRNAs (miRNAs): These small RNA molecules regulate gene expression post-transcriptionally. Changes in miRNA profiles in RA patients have been associated with disease activity and response to therapy.
- Long Non-Coding RNAs (lncRNAs): Emerging evidence suggests that lncRNAs also play roles in the regulation of immune responses in RA. They may serve as potential biomarkers for diagnosis and targets for therapy.
Key Takeaway:
Epigenetic modifications are reversible, which opens the door to novel therapeutic strategies aimed at “resetting” the immune system in RA.
The Microbiome Connection
Emerging research is shining a light on the role of the gut microbiome in the etiology of rheumatoid arthritis:
- Gut Dysbiosis: Studies show that individuals with RA often have an imbalance in their gut bacteria (dysbiosis). Certain bacterial species may influence the immune system, potentially triggering or exacerbating the autoimmune response.
- Molecular Mimicry: Some researchers propose that components of gut bacteria might resemble self-antigens, leading to an immune response that mistakenly targets the body’s own tissues.
- Fungal and Bacterial Triggers: Beyond bacteria, there is interest in whether fungi (such as Candida albicans) might also contribute to RA development through similar mechanisms.
Real-World Example:
A patient with a family history of RA may experience gastrointestinal issues before joint symptoms appear. Analysis of their gut microbiome might reveal a decreased diversity of beneficial bacteria and an overgrowth of pro-inflammatory species. Interventions such as dietary changes, probiotics, or even fecal microbiota transplantation are being explored as potential ways to restore balance and reduce systemic inflammation.
Hormonal and Other Contributing Factors
Sex Differences
- Higher Prevalence in Women: Rheumatoid arthritis affects women two to three times more often than men. Although the reasons are not fully understood, hormonal influences and differences in immune response likely play significant roles.
- Hormonal Fluctuations: Changes in estrogen and progesterone levels during events such as pregnancy, menstruation, and menopause may affect immune system function and influence the onset or progression of RA.
Age and Other Demographics
- Age-Related Risks: RA typically develops in middle age, though it can occur at any age. The risk increases with age, possibly due to cumulative environmental exposures and changes in immune function over time.
- Obesity: Excess body weight is associated with an increased risk of RA, likely because adipose tissue releases pro-inflammatory cytokines that contribute to systemic inflammation.
Gene-Environment Interactions
Perhaps the most compelling aspect of the etiology of rheumatoid arthritis is how genetic predisposition and environmental factors interact:
- The Smoking Connection: Studies consistently show that smoking significantly increases the risk of RA in individuals carrying high-risk HLA-DRB1 alleles. Smoking can promote protein citrullination in the lungs, which in turn may trigger an immune response leading to the production of ACPA.
- Occupational and Environmental Exposures: Exposure to silica dust and other pollutants has been linked to RA, particularly in individuals with susceptible genetic backgrounds.
- Lifestyle and Diet: Diets high in processed foods and low in fiber, as well as obesity, create a pro-inflammatory state that may work synergistically with genetic risk factors.
Illustrative Example:
Consider a middle-aged woman with a strong family history of RA who has been a long-term smoker. Her genetic makeup (including high-risk HLA-DRB1 alleles) predisposes her to RA. Over the years, the inflammatory effects of smoking—through mechanisms like increased protein citrullination—trigger the autoimmune process, ultimately leading to the development of RA. This is a prime example of gene-environment interaction at work.
Real-World Examples and Case Studies
Case Study 1: The Impact of Smoking and Genetics
Imagine two individuals with a family history of RA. Both carry the high-risk shared epitope allele of HLA-DRB1. However, one has a history of heavy smoking, while the other has never smoked. Studies have shown that the smoker’s risk of developing RA is dramatically higher, especially for ACPA-positive RA. This case underscores the powerful interplay between genetics and environmental factors in the etiology of rheumatoid arthritis.
Case Study 2: Early Detection Through Autoantibodies
Many patients with RA show the presence of autoantibodies such as RF and anti-CCP years before joint symptoms appear. For example, a patient in their 30s may feel occasional joint stiffness, and routine blood tests reveal elevated anti-CCP antibodies. Early identification of these markers allows for prompt intervention, which can slow disease progression and reduce long-term joint damage.
Case Study 3: The Role of the Microbiome
Emerging research has illustrated that gut dysbiosis may be linked to the onset of RA. In one study, patients with early RA had reduced gut microbial diversity and an overrepresentation of certain pro-inflammatory bacterial species. Interventions aimed at restoring a healthy microbiome—such as dietary modifications or probiotics—are currently under investigation as potential complementary treatments for RA.
Case Study 4: Epigenetic Modifications in RA
In a clinical study, researchers found that patients with RA exhibited hypomethylation in specific gene promoters associated with the immune response. This epigenetic change was linked to the overexpression of inflammatory cytokines. Such findings not only deepen our understanding of the etiology of rheumatoid arthritis but also open up new therapeutic avenues. Drugs targeting epigenetic modifications are being explored as potential treatments to “reset” the immune system in RA.
Why Understanding the Etiology Matters
Clinical Benefits
- Early Diagnosis and Intervention: Recognizing the genetic, environmental, and immunologic markers early in the disease process can lead to prompt treatment. Early intervention is key to preventing irreversible joint damage.
- Personalized Treatment: A deep understanding of the etiology of rheumatoid arthritis allows clinicians to tailor treatments based on an individual’s unique risk factors. For instance, a patient with a strong genetic predisposition and a history of smoking may benefit from a combination of DMARDs and lifestyle interventions.
- Improved Outcomes: By targeting the underlying causes rather than just the symptoms, new therapeutic approaches may slow or even halt disease progression, significantly improving the quality of life.
Societal and Economic Impact
- Reducing Disability: RA is a leading cause of disability. Early and targeted interventions can reduce the economic burden on healthcare systems and help individuals maintain productivity and independence.
- Patient Education and Empowerment: Understanding the causes of RA enables patients to take an active role in their healthcare. Education about lifestyle modifications—such as smoking cessation, healthy diet, and regular exercise—empowers patients to reduce their risk and manage symptoms effectively.
- Public Health Strategies: Insights into the etiology of rheumatoid arthritis guide public health policies on reducing environmental exposures (e.g., air pollution, occupational hazards) that may trigger RA in susceptible populations.
Research and Innovation
- Biomarker Development: Identifying biomarkers such as autoantibodies and epigenetic changes can help in the early detection and monitoring of RA. These biomarkers also serve as targets for novel therapies.
- New Therapeutic Targets: Advances in our understanding of epigenetics, the microbiome, and immune regulation are paving the way for innovative treatments. For example, drugs that modulate epigenetic marks or restore a healthy gut microbiota are promising areas of research.
- Interdisciplinary Collaboration: The complexity of RA’s etiology encourages collaboration among geneticists, immunologists, epidemiologists, and clinicians. Such interdisciplinary efforts are crucial for developing a holistic approach to RA management.
Common Misconceptions and FAQs
Common Misconceptions
Misconception 1: RA Is Caused Solely by Wear and Tear
Reality: Unlike osteoarthritis, which is due to joint wear and tear, rheumatoid arthritis is an autoimmune disease driven by an aberrant immune response. Its etiology involves genetics, environmental factors, and immune system dysregulation.Misconception 2: RA Is Only a Joint Disease
Reality: Although RA primarily affects joints, it is a systemic disease. The inflammation can impact other organs like the heart, lungs, eyes, and even the skin.Misconception 3: If You Have a Family History, You Will Definitely Get RA
Reality: While having a family history increases your risk, RA does not follow a simple inheritance pattern. Environmental factors such as smoking, infections, and diet also play significant roles.Misconception 4: Autoantibodies Always Indicate RA
Reality: Autoantibodies such as RF and anti-CCP are important markers for RA, but they can occasionally be found in other conditions or even in healthy individuals. Their presence must be interpreted alongside clinical findings.
Frequently Asked Questions (FAQs)
Q1: What does “etiology” mean in the context of rheumatoid arthritis?
A: It refers to the study of the underlying causes and factors that lead to the development of RA, including genetic, environmental, immunologic, and epigenetic influences.
Q2: How do genetics contribute to RA?
A: Certain genes, particularly variations in the HLA-DRB1 gene, increase susceptibility to RA. Twin and family studies support a strong genetic component, although not everyone with these genes will develop RA.
Q3: Can lifestyle changes help prevent RA?
A: Yes. Avoiding risk factors such as smoking, maintaining a healthy weight, eating a balanced diet rich in anti-inflammatory nutrients, and reducing exposure to pollutants may help lower the risk in genetically predisposed individuals.
Q4: Why are autoantibodies important in RA?
A: Autoantibodies like RF and anti-CCP can appear years before clinical symptoms and are useful in early diagnosis and predicting disease progression, though they are not exclusively found in RA.
Q5: What is the role of the microbiome in RA?
A: Emerging research suggests that imbalances in gut bacteria (dysbiosis) may trigger or exacerbate immune responses, contributing to the onset and progression of RA.
Q6: How do epigenetic factors influence RA?
A: Epigenetic modifications such as DNA methylation and histone modifications alter gene expression without changing the DNA sequence. These changes can lead to an overactive immune response, playing a role in RA development.
Modern Relevance and Current Trends
Advances in Genetic Research
Recent technological advances such as high-throughput sequencing and genome-wide association studies (GWAS) have identified multiple genetic loci linked to RA. Researchers are now:
- Mapping out the complex network of genes that contribute to RA.
- Investigating how specific genetic variants interact with environmental triggers to set off the autoimmune process.
- Using genetic information to develop personalized medicine approaches that tailor treatments to an individual’s genetic makeup.
Epigenetics and Therapeutic Innovations
Epigenetics is one of the most exciting areas of current RA research:
- Reversible Changes: Unlike genetic mutations, epigenetic changes are reversible. This opens the possibility of developing drugs that “reset” abnormal gene expression.
- New Drug Targets: Histone deacetylase (HDAC) inhibitors and DNA methyltransferase inhibitors are being investigated as potential treatments to modulate the immune response in RA.
- Biomarker Discovery: Epigenetic markers may soon serve as biomarkers for early diagnosis, disease activity, and response to therapy.
The Microbiome: A New Frontier
Research on the gut microbiome has revealed that:
- Gut Dysbiosis: Changes in the balance of gut bacteria are linked to systemic inflammation in RA.
- Potential Interventions: Strategies such as probiotics, prebiotics, and even fecal microbiota transplantation are being explored as ways to restore a healthy microbiome and reduce RA symptoms.
- Mechanistic Insights: Studies are uncovering how microbial metabolites may influence immune cell function, further clarifying the complex etiology of RA.
Environmental and Lifestyle Interventions
Public health initiatives are increasingly focusing on modifiable risk factors:
- Smoking Cessation Programs: Given the strong link between smoking and RA, efforts to reduce tobacco use can have a significant impact.
- Diet and Nutrition: Nutritional interventions that promote an anti-inflammatory diet—rich in omega-3 fatty acids, antioxidants, and fiber—are being studied for their potential to reduce RA risk and severity.
- Occupational Safety: Reducing exposure to harmful substances (such as silica and asbestos) in the workplace is another important preventive strategy.
Immunologic and Autoimmune Mechanism Discoveries
The development of biologic drugs that target specific cytokines (such as TNF-α and IL-6) has transformed RA treatment:
- Precision Therapies: Biologic DMARDs (disease-modifying antirheumatic drugs) are now a mainstay in the management of RA, significantly improving outcomes for many patients.
- Ongoing Research: Newer agents are under investigation to further refine these treatments and minimize side effects.
- Integration with Other Therapies: Combining biologics with traditional DMARDs and lifestyle interventions holds promise for even better disease control.
Modern Diagnostic Tools
Advances in medical imaging and laboratory testing have improved our ability to diagnose RA early:
- Ultrasound and MRI: These imaging techniques can detect joint inflammation and structural changes before they are visible on X-rays.
- Biomarker Panels: Comprehensive panels that include genetic, epigenetic, and serological markers are being developed to predict disease onset and monitor progression.
- Digital Health: Wearable devices and mobile health apps are increasingly used to monitor symptoms and treatment responses in real time.
Conclusion and Call to Action
Understanding the etiology of rheumatoid arthritis is crucial for unlocking better ways to diagnose, treat, and ultimately prevent this debilitating disease. By exploring the intricate interplay of genetic predispositions, environmental triggers, autoimmune mechanisms, epigenetic modifications, and the gut microbiome, researchers are piecing together the puzzle of RA’s origins. This holistic understanding not only informs more personalized treatment strategies but also paves the way for novel interventions that may one day prevent RA from developing at all.
Key Takeaways:
- Multifactorial Origins: RA is caused by a complex mix of genetic, environmental, immunologic, and epigenetic factors.
- Importance of Early Detection: Autoantibodies such as RF and anti-CCP can serve as early markers, allowing for timely intervention.
- Lifestyle Matters: Modifiable factors like smoking cessation, diet, and stress management are critical in reducing RA risk.
- Cutting-Edge Research: Advances in genomics, epigenetics, and microbiome studies are revolutionizing our understanding and treatment of RA.
- Personalized Medicine: Insights from ongoing research are paving the way for tailored therapies that target the specific causes of RA in each patient.
Call to Action
If you found this guide helpful, please share it with friends, family, and anyone who might benefit from understanding the etiology of rheumatoid arthritis. Whether you’re a patient seeking more insight into your condition or a caregiver looking for ways to help, staying informed is the first step toward better management and improved quality of life.
For more information and the latest updates on rheumatoid arthritis, check out reputable sources such as the World Health Organization, Mayo Clinic, and MedlinePlus. We encourage you to subscribe to our newsletter, leave your comments below, and join our community to continue the conversation about how we can work together to conquer rheumatoid arthritis.
Final Thoughts
The etiology of rheumatoid arthritis is a fascinating and ever-evolving field. With ongoing research shining new light on the genetic, environmental, and immune pathways involved, we are gradually moving toward a future where early detection and personalized treatment can transform the lives of those affected by RA. Embrace the power of knowledge—by understanding the roots of rheumatoid arthritis, we empower ourselves to fight back against its debilitating effects.
Stay informed, stay proactive, and together we can pave the way toward better health and a brighter future for all those impacted by rheumatoid arthritis.