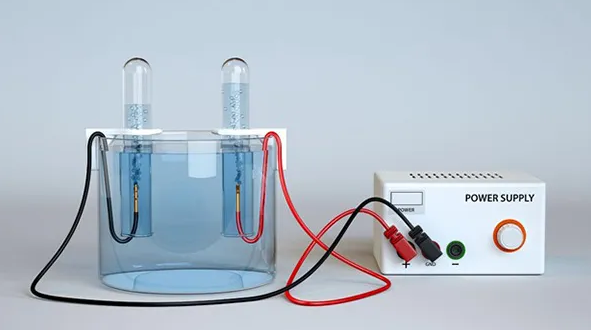
Have you ever wondered how water can be split into hydrogen and oxygen, or how metals are extracted from their ores using electricity? Perhaps you've seen electrodes at work in a science lab experiment or read about hydrogen fuel cells promising a greener future. These processes, which might seem like magic at first glance, are powered by a fascinating chemical phenomenon known as electrolysis.
In our modern world, where sustainable energy and advanced manufacturing are more critical than ever, understanding electrolysis is key to appreciating the role that electricity plays in driving non-spontaneous chemical reactions.
A Straightforward Definition
Core Definition
Electrolysis is a chemical process that uses electrical energy to drive a non-spontaneous chemical reaction. In simpler terms, it is the method of decomposing a compound—typically an electrolyte solution or molten salt—into its constituent elements or simpler compounds by applying an electric current.
For example, when you apply an electrical current to water, it breaks down into hydrogen gas and oxygen gas. This process is described by the following chemical equation:
Essential Characteristics
- Requires an External Power Source:
Electrolysis is driven by an external electrical current, making it fundamentally different from spontaneous chemical reactions. - Uses a Semi-Permeable Medium:
The process takes place in an electrolyte—a substance (often a liquid solution or molten salt) that contains free ions—and between two electrodes (an anode and a cathode). - Non-Spontaneous Reaction:
The reaction induced by electrolysis does not occur on its own; it requires the input of electrical energy to proceed. - Production of Gases and Elements:
In many cases, electrolysis results in the formation of gases (such as hydrogen and oxygen from water) or the extraction of pure metals from their ores.
Historical and Contextual Background
18th Century
Scientists like Benjamin Franklin and Luigi Galvani explored the effects of electricity on various substances, laying groundwork for future discoveries.
1830s
Michael Faraday formulated the laws of electrolysis, which quantitatively described the relationship between electric charge and chemical change.
Late 19th Century
The Hall-Héroult process for aluminum extraction revolutionized the metal industry by making aluminum production more efficient.
20th Century
Advancements in electrical engineering and materials science improved electrolysis techniques and expanded applications into water treatment and medical fields.
21st Century
Focus on renewable energy has made electrolysis central to producing green hydrogen and developing sustainable technologies.
Faraday's First Law
The amount of chemical change produced at an electrode is proportional to the quantity of electricity passed through the electrolyte.
Faraday's Second Law
The amounts of different substances produced by the same quantity of electricity passing through the electrolytic solution are proportional to their equivalent weights.
These laws allow for the precise calculation of the material changes that occur during electrolysis and underpin many industrial applications.
The Science Behind Electrolysis
Fundamental Principles
Electrolysis is based on the principle that an electric current can drive a chemical reaction that would not occur spontaneously. This process involves:
Electrolyte
A substance—typically a liquid solution or molten compound—that contains ions, which are charged particles that can move freely.
Electrodes
Two conductors, an anode (positive electrode) and a cathode (negative electrode), are immersed in the electrolyte. When an electrical current is applied, ions in the electrolyte move toward the electrodes to undergo chemical changes.
Electrical Current
A power source supplies the necessary energy to initiate the non-spontaneous reaction. The direction of the current determines the reactions at the anode and cathode.
Types of Electrolysis
Overview
Water electrolysis is the process of decomposing water (H₂O) into hydrogen (H₂) and oxygen (O₂) gases by applying an electric current.
Chemical Reaction
Applications
Water electrolysis is central to the production of hydrogen fuel, which is considered a clean and renewable energy source when powered by renewable energy.
Overview
Electroplating involves depositing a thin layer of metal onto the surface of an object by using an electrical current.
Process
The object to be plated is connected as the cathode in an electrolytic solution containing metal ions. When current is applied, metal ions are reduced and deposit on the object's surface.
Applications
This technique is widely used for corrosion protection, decorative finishes, and improving surface properties of materials.
Overview
Electrolysis is used to extract and refine metals from their ores. One of the most famous examples is the extraction of aluminum using the Hall-Héroult process.
Process
In the Hall-Héroult process, aluminum oxide (Al₂O₃) is dissolved in molten cryolite and subjected to electrolysis, resulting in the production of pure aluminum.
Applications
This method is essential for producing high-purity metals used in aerospace, automotive, and consumer electronics industries.
Overview
Electrolysis can drive chemical reactions to synthesize various organic and inorganic compounds. It is used in processes such as the production of chlorine and sodium hydroxide from saltwater.
Applications
In the chemical industry, electrolysis is critical for producing a wide range of compounds essential for manufacturing, pharmaceuticals, and everyday products.
Addressing Common Misconceptions and FAQs
Misconception 1: Electrolysis Is the Same as Regular Chemical Reactions
Electrolysis is unique because it requires electrical energy to drive a non-spontaneous reaction. Unlike spontaneous reactions that occur naturally, electrolysis needs an external power source to force the reaction to proceed.
Misconception 2: Electrolysis Only Applies to Water
While water electrolysis is one of the most well-known applications, the process applies to a wide range of substances. Electrolysis is used in the extraction of metals, electroplating, chemical synthesis, and even in developing new materials and sensors.
Misconception 3: Electrolysis Is Inefficient and Too Expensive
Technological advancements have significantly improved the efficiency and reduced the cost of electrolysis systems. With the integration of renewable energy sources and advanced catalysts, modern electrolysis is increasingly cost-effective and scalable.
Test Your Knowledge
Real-World Applications and Case Studies
Industrial Applications
Hydrogen Production
Electrolysis is a key technology in the production of hydrogen fuel. Companies worldwide are investing in advanced electrolyzer systems to produce "green hydrogen" using renewable energy sources.
Metal Refining
The extraction and purification of metals like aluminum and copper rely on electrolysis. For instance, the Hall-Héroult process for aluminum extraction has transformed the metal industry by making aluminum production more efficient and cost-effective.
Electroplating
Industries such as automotive and electronics use electroplating to enhance the durability, appearance, and corrosion resistance of their products.
Environmental and Sustainable Technologies
Water Treatment
Electrolysis is employed in wastewater treatment to break down pollutants and recover valuable materials from industrial effluents.
Renewable Energy Integration
As the world shifts toward renewable energy, electrolysis is increasingly used to produce hydrogen—a clean fuel that can be used for transportation, power generation, and industrial processes.
Medical and Consumer Applications
Hair Removal
In the cosmetic and medical fields, electrolysis is a method used for permanent hair removal. It involves inserting a tiny probe into hair follicles and applying an electrical current to destroy the follicle.
Electrochemical Sensors
Electrolysis principles underpin the operation of various sensors, including those used in medical diagnostics, environmental monitoring, and food safety testing.
Modern Relevance and Current Trends
The Push for Renewable Energy
Green Hydrogen Production: Electrolysis is at the forefront of renewable energy initiatives, especially in the production of green hydrogen. Powered by renewable energy sources like wind and solar, water electrolysis can produce hydrogen fuel with minimal environmental impact.
Energy Transition: Governments and industries worldwide are investing in electrolysis technology as part of the broader energy transition from fossil fuels to cleaner, renewable sources.
Technological Innovations
Advanced Electrolyzers: Research and development in the field of electrolysis have led to the creation of more efficient and durable electrolyzers. New materials and catalyst technologies are improving the conversion efficiency and lowering the cost of hydrogen production.
Integration with Smart Grids: Electrolysis systems are increasingly being integrated with smart grids and IoT devices, enabling real-time monitoring, predictive maintenance, and optimized energy management.
Practical Tips for Leveraging Electrolysis
- Deep Dive into Fundamentals:
- Study the underlying chemical principles of electrolysis, including Faraday's laws and the role of electrodes, electrolytes, and current.
- Use interactive simulations, such as those available on PhET Interactive Simulations, to visualize the process.
- Engage in Laboratory Experiments:
- If possible, conduct simple electrolysis experiments (like water splitting) to see the process in action. Hands-on learning reinforces theoretical concepts.
- Utilize Online Courses and Tutorials:
- Platforms like Coursera, edX, and Khan Academy offer courses on chemistry and electrochemistry that cover electrolysis in detail.
- Adopt Cutting-Edge Technologies:
- Stay informed about the latest developments in electrolyzer technology and renewable energy integration. Implementing advanced electrolysis systems can improve efficiency and reduce costs.
- Invest in Employee Training:
- Ensure that your team is well-versed in the principles and applications of electrolysis. Regular training can help optimize processes and enhance overall productivity.
- Leverage Data Analytics:
- Use real-time monitoring and data analysis tools to track the performance of electrolysis systems. This can help identify areas for improvement and ensure the reliability of your processes.
- Stay Informed:
- Follow reputable science blogs, journals, and news outlets to keep up with the latest research on electrolysis. Websites like Science Daily and Phys.org offer regular updates on advancements in electrochemistry.
- Join Online Communities:
- Participate in forums such as Reddit's r/chemistry or Stack Exchange's Chemistry section to ask questions, share insights, and learn from experts.
- Experiment at Home:
- Simple DIY electrolysis projects can be both educational and fun. Always follow safety guidelines and conduct experiments in a controlled environment.
Interactive Elements
Electrolysis Calculator
Faraday's Law Calculator
Calculate the mass of substance produced during electrolysis based on Faraday's Law:
Water Electrolysis Simulation
Electroplating Simulation
Coming soon! Our interactive electroplating simulation will allow you to visualize how metal ions are deposited onto objects during the electroplating process.
Common Types of Electrolysis
| Type | Electrolyte | Products | Applications |
|---|---|---|---|
| Water Electrolysis | Water (with salts) | Hydrogen and Oxygen gases | Hydrogen fuel production, Energy storage |
| Molten Salt Electrolysis | Molten metal salts | Pure metals | Aluminum, sodium, and magnesium production |
| Electroplating | Metal salt solutions | Metal coatings | Jewelry, automotive parts, electronics |
| Chlor-alkali Process | Brine (NaCl solution) | Chlorine, hydrogen, sodium hydroxide | Chemical industry, bleach production |
Additional Resources
Official and Educational Websites
- Khan Academy – Electrolysis – Explore lessons on electrolysis and related chemical processes.
- Coursera and edX – Offer courses in chemistry, renewable energy, and electrochemical engineering.
Books and Academic Texts
- Electrochemical Methods: Fundamentals and Applications by Allen J. Bard and Larry R. Faulkner – A comprehensive guide to the science of electrochemistry, including electrolysis.
- Renewable Hydrogen Production: Principles and Technologies – Focuses on water electrolysis and its role in sustainable energy.
Research Journals and Articles
- Journal of The Electrochemical Society – For the latest research on electrolysis and related electrochemical processes.
- International Journal of Hydrogen Energy – Offers studies on hydrogen production via electrolysis and its applications in renewable energy.
Industry Reports and Case Studies
- International Energy Agency (IEA) – Provides reports and statistics on renewable energy and hydrogen production.
- World Economic Forum (WEF) – Features articles and case studies on sustainable energy and technological innovations.
Digital Tools and Simulations
- PhET Interactive Simulations – Offers interactive simulations on chemical processes, including electrolysis.
- Electrolyzer Efficiency Calculators – Online tools to help analyze the performance and efficiency of different electrolysis systems.
Community Forums and Discussion Groups
- Reddit: r/chemistry – Engage with a community of chemists discussing electrolysis and other chemical phenomena.
- Stack Exchange: Chemistry – A Q&A forum for in-depth discussions on electrolysis, electrochemical reactions, and related topics.
Conclusion
Summarizing the Key Points
- Definition: Electrolysis is a chemical process that uses electrical energy to drive a non-spontaneous reaction, typically decomposing a compound into its constituent elements.
- Core Principles: Electrolysis relies on an electrolyte, electrodes (anode and cathode), and an external power source to force chemical reactions. Faraday's laws provide the quantitative foundation for understanding this process.
- Historical Evolution: From early experiments by pioneers like Michael Faraday to modern applications in renewable energy and industrial processing, electrolysis has evolved into a critical technology in science and industry.
- Diverse Applications: Electrolysis is used in a variety of fields including hydrogen production, metal extraction, electroplating, wastewater treatment, and even medical procedures. Its role in advancing renewable energy and sustainable practices is especially significant.
- Modern Trends: With the integration of AI, smart technologies, and increased global focus on sustainability, electrolysis continues to evolve, making it more efficient and accessible for a wide range of applications.
- Benefits: The process of electrolysis not only drives technological innovation but also contributes to environmental protection, economic efficiency, and the advancement of scientific knowledge.
Final Thoughts
From splitting water into hydrogen and oxygen to refining metals and advancing renewable energy, electrolysis is a cornerstone process that continues to drive innovation and sustainability in our modern world. Understanding what is electrolysis provides invaluable insights into the chemical processes that power so many technologies and industries. Whether you're a student, scientist, or industry professional, embracing the principles of electrolysis can empower you to contribute to a cleaner, more efficient, and sustainable future.
Embrace the journey of discovery, share your insights, and let the power of electrolysis inspire you to push the boundaries of what's possible. Happy experimenting, and may your pursuit of knowledge electrify your world!