7.9 Introduction to Le Châtelier’s Principle: Controlling Equilibrium Shifts
How Can We Control Equilibrium?
So far, our focus has been on reactions reaching equilibrium and staying there. But what if we want to change the course of a reaction? By understanding Le Châtelier’s Principle, we can predict how a chemical system at equilibrium responds to various changes or “stressors.” This principle is vital for mastering chemical reactions and their behavior under different conditions, making it a must-know for AP Chemistry!
What Is Le Châtelier’s Principle?
Le Châtelier’s Principle states: “If a dynamic equilibrium is disturbed by changing conditions, the system responds by shifting the equilibrium position to counteract the disturbance and reestablish equilibrium.” To break this down:
- Dynamic Equilibrium: This means the forward and reverse reactions occur at equal rates, so the concentrations of reactants and products remain constant.
- Stressors: Changes in concentration, temperature, or pressure that disrupt the equilibrium.
- System Response: The system shifts to counterbalance the change and restore equilibrium.
Think of it as a seesaw—adding weight on one side makes the system shift to maintain balance.
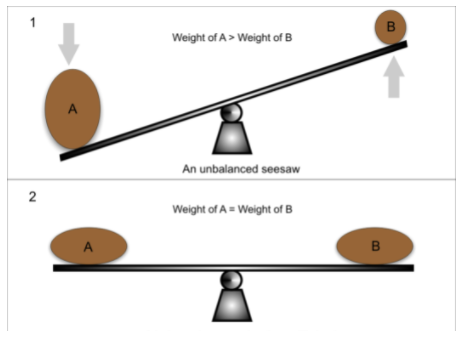
Image Courtesy of Greater Minds Tutors
Factors That Influence Equilibrium
1. Concentration Changes
Changing the concentration of a reactant or product disrupts the equilibrium balance. Here’s how it works:
- Increasing Reactants: The system shifts to consume excess reactants and produce more products.
- Increasing Products: The system shifts to consume excess products and form more reactants.
- Decreasing Reactants or Products: The system shifts to produce more of the depleted substance to balance the loss.
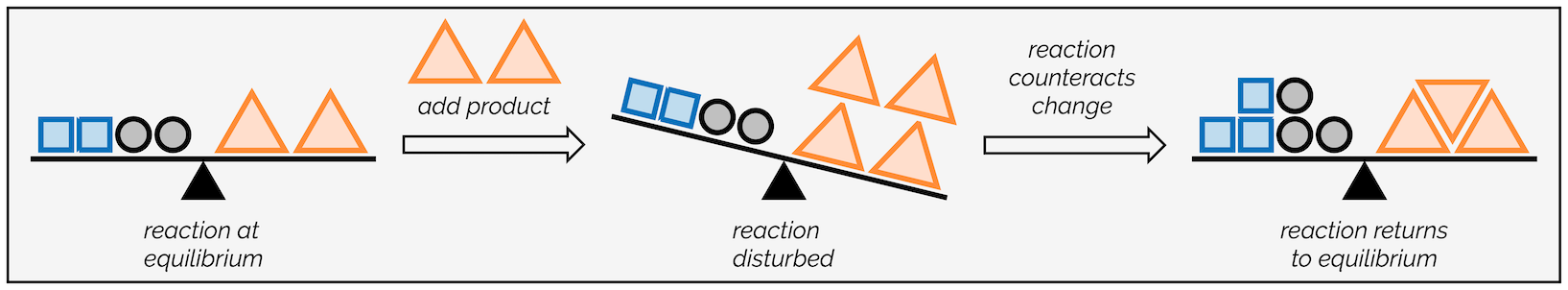
Image Courtesy of Making Molecules
Example:
Consider the reaction:
Fe³⁺ + SCN⁻ ⇌ FeSCN²⁺
If you add more SCN⁻, the system shifts right to produce more FeSCN²⁺. Conversely, removing Fe³⁺ causes the system to shift left, producing more Fe³⁺ and SCN⁻ to balance the change.
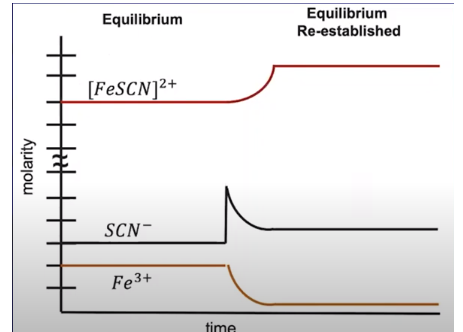
Image From Abigail Giordano
2. Temperature Changes
The effect of temperature depends on whether the reaction is exothermic (releases heat) or endothermic (absorbs heat):
- Exothermic Reactions (ΔH° < 0): Increasing temperature shifts equilibrium left (toward reactants) to absorb excess heat.
- Endothermic Reactions (ΔH° > 0): Increasing temperature shifts equilibrium right (toward products) to consume added heat.
Example:
N₂ + 3H₂ ⇌ 2NH₃ (ΔH° = -92 kJ/mol)
Since this reaction is exothermic, adding heat shifts equilibrium left, forming more reactants.
3. Pressure Changes (for Gaseous Systems)
When pressure changes, the system responds based on the number of moles of gas:
- Increased Pressure: The equilibrium shifts toward the side with fewer moles of gas.
- Decreased Pressure: The equilibrium shifts toward the side with more moles of gas.
Note: Inert gases (e.g., helium) added to the system do not affect equilibrium.
Quick Reference Table: Le Châtelier’s Principle
| Stress | Shift | Explanation |
|---|---|---|
| Increase concentration of a substance | Away from the substance | Extra concentration needs to be used up |
| Decrease concentration of a substance | Toward the substance | More of the substance needs to be produced |
| Increase pressure (gaseous system) | Toward fewer moles of gas | Higher pressure favors lower volume |
| Decrease pressure (gaseous system) | Toward more moles of gas | Lower pressure favors higher volume |
| Increase temperature | Favors endothermic reaction | Heat must be absorbed |
| Decrease temperature | Favors exothermic reaction | Heat must be produced |
| Add a catalyst | — no shift — | Increases rate of both forward and reverse reactions equally |
Practical Applications of Le Châtelier’s Principle
- Industrial Processes: Understanding how to shift reactions helps optimize product yields (e.g., ammonia synthesis in the Haber process).
- Environmental Chemistry: Helps predict how pollutants react in the atmosphere.
- Biological Systems: Many biochemical reactions are in dynamic equilibrium, responding to changes in conditions (e.g., enzyme activity regulation).
Real-World Example: The Haber Process
Reaction:
N₂ + 3H₂ ⇌ 2NH₃ (exothermic)
Increased pressure shifts equilibrium toward the formation of NH₃. High temperatures, however, shift it toward reactants. By adjusting temperature and pressure carefully, industrial processes maximize ammonia production.
Mastering Equilibrium with Le Châtelier
Understanding Le Châtelier’s Principle empowers you to predict how equilibrium systems will react to changes in concentration, temperature, or pressure. Mastering this concept is essential for success on the AP Chemistry exam and provides valuable insight into the behavior of chemical systems in real-life applications.







