7.3 Reaction Quotient and Equilibrium Constant
Introduction to Reaction Quotient (Q)
So far, we’ve focused on reactions at equilibrium, but what about reactions that are not quite there yet? The reaction quotient (Q) is a valuable tool that allows us to determine the progress of a chemical reaction at any point. It helps us predict the direction a reaction will shift to reach equilibrium by comparing the concentrations of reactants and products at a specific moment in time.
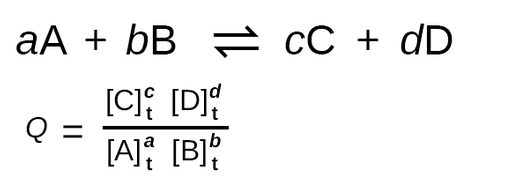
Image From Labster
What is the Reaction Quotient?
In essence, Q measures the relative concentrations of products and reactants in a reaction at any given time. To calculate Q, we use the same formula as we do for the equilibrium constant (K), but there is a crucial difference: while K is calculated using concentrations at equilibrium, Q is calculated using concentrations at any point in time.
The Formula for Q and K
The formulas for Q and K are nearly identical:
- Q uses concentrations at a specific time, denoted as [A]_t, [B]_t, [C]_t, and so on.
- K uses equilibrium concentrations.
This distinction makes Q an essential tool for predicting whether a reaction will proceed forward, reverse, or remain at equilibrium.
Understanding Equilibrium vs. Non-Equilibrium Concentrations
Reactions will naturally shift toward equilibrium to minimize their potential energy and achieve stability. When a reaction is at equilibrium, the concentrations of reactants and products remain constant because the forward and reverse reaction rates are equal. However, when a reaction is not at equilibrium, it will shift to reach equilibrium by adjusting the concentrations of reactants and products.
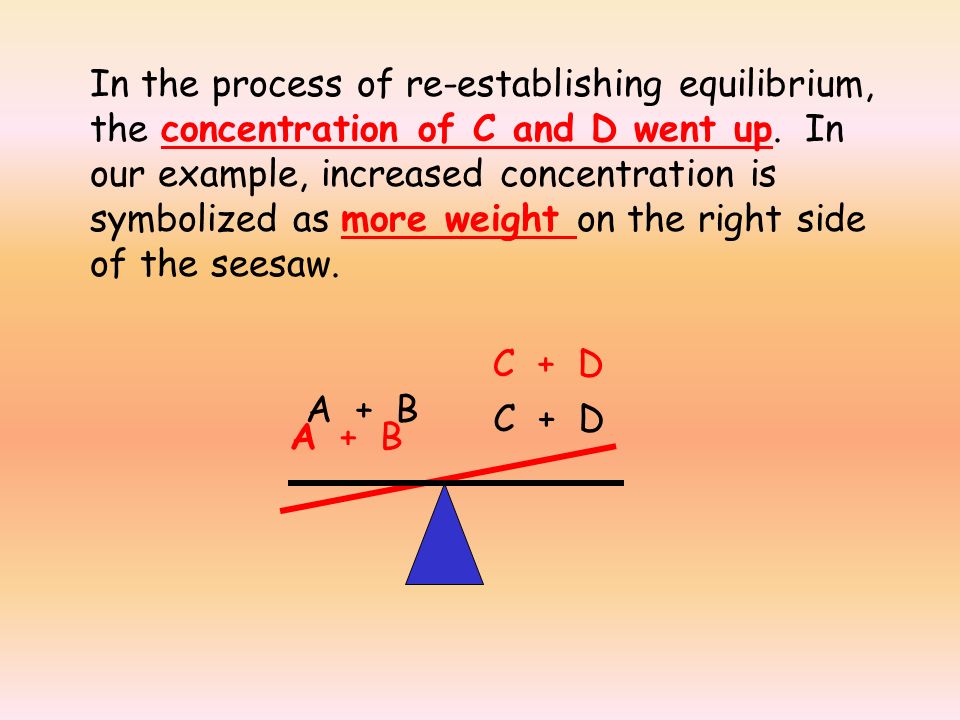
This adjustment process can be visualized like a seesaw. If one side has too much weight (too many products or reactants), the reaction will adjust itself to restore balance.
Comparing Q and K: The Three Scenarios
To determine the direction a reaction will proceed, we compare Q to K. There are three possibilities:
Q > K
When Q is greater than K, the concentration of products is higher than what is expected at equilibrium. The reaction is in a post-equilibrium state, meaning it will shift to the left to convert excess products into reactants until equilibrium is restored.👉 The reaction moves toward reactants.
Q = K
When Q equals K, the reaction is at equilibrium. There will be no net change in the concentrations of reactants or products.⚖️ The reaction is at equilibrium.
Q < K
When Q is less than K, the concentration of reactants is higher than what is expected at equilibrium. The reaction is in a pre-equilibrium state, so it will shift to the right to produce more products.👉 The reaction moves toward products.
Visualizing Q vs. K
Here’s a helpful way to visualize how Q moves toward K: if Q is too high, the reaction will shift left (toward reactants) to lower Q. If Q is too low, the reaction will shift right (toward products) to increase Q.
Image Courtesy of LibreTexts/Equilibria/Chemical_Equilibria/Difference_Between_K_And_Q)
Mathematical Explanation
Let’s consider the reaction A + B ⇌ C + D with Q calculated as follows:
Q = [C]_t[D]_t / [A]_t[B]_t
- If Q > K, [C]_t and [D]_t are too high, so the reaction shifts left to produce more reactants.
- If Q < K, [A]_t and [B]_t are too high, so the reaction shifts right to produce more products.
Image Courtesy of Kathlyn Parrish
Practicing Q vs. K with an Example
Consider the reaction:
2NOBr ⇌ 2NO + Br₂
Given: K_c = 0.0142, and the initial concentrations are [NOBr] = 1.0 M, [NO] = 0.2 M, and [Br₂] = 0.8 M. Will the reaction shift left or right to reach equilibrium?
Calculate Q:
Q = [NO]²[Br₂] / [NOBr]²
Q = (0.2)²(0.8) / (1)² = 0.032Compare Q to K:
Since Q > K (0.032 > 0.0142), the reaction will shift to the left to decrease the concentration of products and increase the concentration of reactants.
Conclusion
Understanding the relationship between Q and K provides valuable insight into the behavior of chemical reactions. By comparing Q to K, we can predict the direction in which a reaction will proceed, making it a powerful tool for chemists and students alike.