7.2 Direction of Reversible Reactions
Review of Reversible Reactions and Equilibrium
Welcome to another dive into the fascinating world of chemical equilibrium! In this segment, we explore the directionality of reversible reactions, a crucial component in understanding how chemical systems reach and maintain equilibrium. As we learned, equilibrium is the point at which the rates of forward (A → B) and backward (B → A) reactions equalize, leading to a state where concentrations of reactants and products remain constant. This is symbolized by a double arrow (⇌), indicating that both reactions occur continuously.
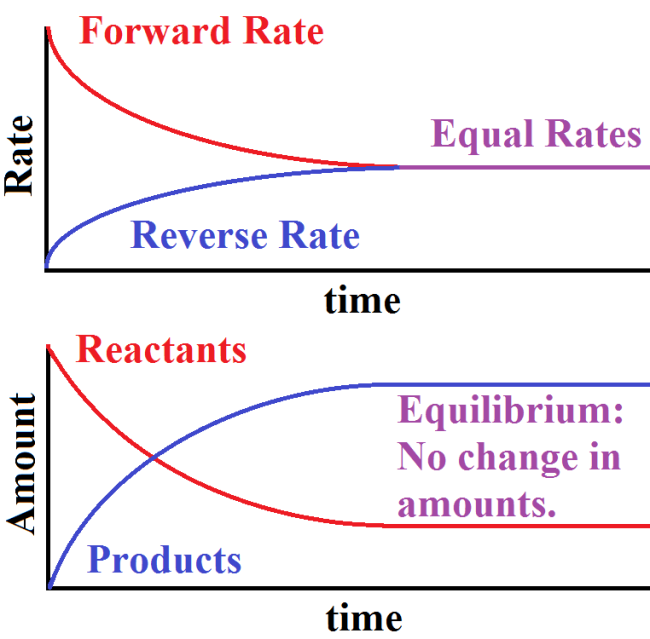
Image From EduBlog
Equilibrium Graphically Represented
To understand equilibrium, it’s helpful to visualize how reaction rates change over time. Initially, the forward reaction (A → B) proceeds rapidly as reactants are plentiful. Over time, as products accumulate, the reverse reaction (B → A) speeds up. Eventually, both reactions reach a state where their rates are equal. At this point, the concentrations of reactants and products become stable, even though reactions continue to occur dynamically.
Graphically, we see this as two converging lines representing the forward and reverse reaction rates, with a plateau indicating equilibrium.
The Favored Direction of Reactions
One key aspect of equilibrium is determining whether a reaction is product-favored or reactant-favored. This tells us which side of the reaction—products or reactants—is predominant at equilibrium:
- Product-Favored Reactions: These reactions result in more products at equilibrium. Mathematically, if the equilibrium constant K is greater than 1, the reaction favors product formation.
- Reactant-Favored Reactions: Conversely, if K is less than 1, the reaction favors the reactants, meaning fewer products form.
- Neutral Reactions: If K is equal to 1, there is an equal distribution of reactants and products at equilibrium.
Example Scenarios:
Reactant-Favored Reaction:
N₂O₄ ⇌ 2NO₂, where K = 4.65 × 10⁻³.
Since K < 1, this reaction is reactant-favored, indicating that at equilibrium, most of the mixture consists of N₂O₄ with minimal NO₂ formation.Product-Favored Reaction:
2O₃ ⇌ 3O₂, where K = 2.5 × 10¹².
Here, K > 1, meaning that this reaction is strongly product-favored, with a large amount of O₂ produced relative to O₃ at equilibrium.
What Does a Reaction’s Direction Tell Us?
Knowing whether a reaction is product-favored or reactant-favored offers valuable insight into the reaction’s behavior:
- High K Value: Indicates a high conversion of reactants to products, suggesting a strongly product-favored reaction.
- Low K Value: Suggests a predominance of reactants at equilibrium, with limited product formation.
Real-Life Example: Acids and Bases
Consider acetic acid (CH₃COOH) and carbonic acid (H₂CO₃). The reactions are as follows:
- Acetic Acid: CH₃COOH ⇌ CH₃COO⁻ + H⁺, K = 1.8 × 10⁻⁵
- Carbonic Acid: H₂CO₃ ⇌ H⁺ + HCO₃⁻, K = 4.3 × 10⁻⁷
Since acetic acid has a higher K value, it dissociates more extensively than carbonic acid. Thus, at equal concentrations, acetic acid will produce more H⁺ ions, making it a stronger acid.
Conclusion
The directionality of reversible reactions and equilibrium constants provides a powerful framework for predicting and explaining chemical behaviors. By understanding whether a reaction is product- or reactant-favored, we gain deeper insights into how far a reaction proceeds and how equilibrium can be influenced by changes in concentration, pressure, and temperature. In the next section, we’ll explore Q, the reaction quotient, and how it helps predict shifts in equilibrium!







