AP Biology 1.5: Structure and Function of Biological Macromolecules
Welcome to AP Biology Unit 1.5! In this section, we explore the structure and function of biological macromolecules. Understanding how the structure of these macromolecules determines their function is fundamental to understanding biology. 🌱
Four Types of Macromolecules: A Refresher
There are four main types of macromolecules: nucleic acids, carbohydrates, lipids, and proteins. These macromolecules are the building blocks of cells and perform a wide range of functions in living organisms.
Nucleic Acids: Made up of nitrogenous bases, sugars, and phosphate groups. DNA stores genetic information, while RNA carries out DNA’s instructions and helps synthesize proteins.
Carbohydrates: Composed of carbon, hydrogen, and oxygen atoms. Serve as a source of energy and provide structural support. Examples include sugars, starches, and cellulose.
Proteins: Made up of amino acids. Perform a variety of functions, including catalyzing chemical reactions, transporting molecules, and providing structural support.
Lipids: Composed of carbon, hydrogen, and oxygen atoms. Important for energy storage and cell membrane structure. Examples include fats, oils, and phospholipids.
Structure Determines Function
The monomers that make up a polymer play a crucial role in determining the structure and function of the macromolecule. The way monomers are arranged and bonded together affects the overall shape and conformation of the polymer, which, in turn, determines its function.
For example, in a protein, the primary structure (sequence of amino acids) determines the tertiary structure (3D shape), which ultimately determines the protein’s function. Similarly, the arrangement of monomers in a carbohydrate or a nucleic acid affects its structure and function.
Nucleic Acids 🧬
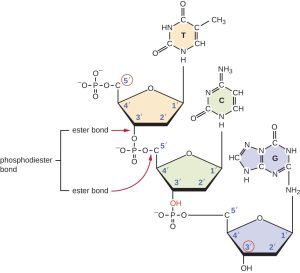
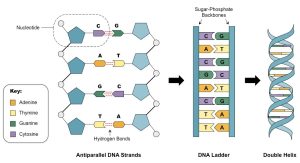
Nucleic acids, such as DNA and RNA, are polymers made up of nucleotide monomers. Nucleotides are linked by covalent bonds between the sugar and phosphate groups of adjacent nucleotides, forming a sugar-phosphate backbone. The sequence of nucleotides carries genetic information that is used to build and maintain living organisms.
The sequence is read from the 5′ end to the 3′ end and is important for processes like DNA replication and RNA transcription. During these processes, nucleotides are added to the 3′ end of the growing strand.
DNA is a double-stranded helix with antiparallel strands held together by hydrogen bonds between complementary bases (A pairs with T, C pairs with G). The specific base pairing ensures stability and accurate genetic information transfer.
Proteins 🥩
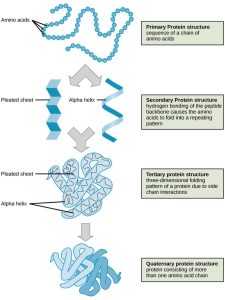
Proteins are linear polymers of amino acids linked by peptide bonds. The primary structure of a protein (its amino acid sequence) determines the tertiary structure (3D shape), which in turn determines the protein’s function.
Levels of Protein Structure
Primary Structure: The sequence of amino acids linked by peptide bonds.
Secondary Structure: Hydrogen bonding between components of the polypeptide backbone forms alpha helices or beta-pleated sheets.
Tertiary Structure: The 3D shape formed by interactions between the alpha helices and beta sheets, including van der Waals forces, hydrophobic interactions, hydrogen bonding, and disulfide bridges.
Quaternary Structure: Interactions between two or more polypeptide chains to form a multi-subunit protein (e.g., hemoglobin).
Denaturation is the process in which a protein loses its structure and function due to the disruption of non-covalent interactions. This can be caused by heat, pH changes, or chemicals. Denatured proteins lose their biological activity.
Carbohydrates 🍩
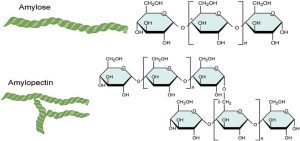
Carbohydrates can be simple (monosaccharides) or complex (polysaccharides). The structure of a carbohydrate can be linear or branched, which affects its properties and function.
Disaccharides and Polysaccharides
Disaccharide: Two monosaccharides joined by a covalent bond through dehydration synthesis. Examples include maltose, sucrose, and lactose.
Polysaccharides: Polymers of sugars that serve storage or structural functions. Examples include:
Starch: Stores energy in plants.
Glycogen: Stores energy in animals (in muscle and liver cells).
Cellulose: Provides structural support in plant cell walls.
Chitin: Provides structural support in the exoskeletons of arthropods and cell walls of fungi.
Study Resources
For more in-depth exploration, check out the AP Bio Unit 1 replays or watch the 2021 Unit 1 Cram session. Understanding how the structure of macromolecules determines their function is a key concept in biology and will help you understand many processes that occur in living organisms.